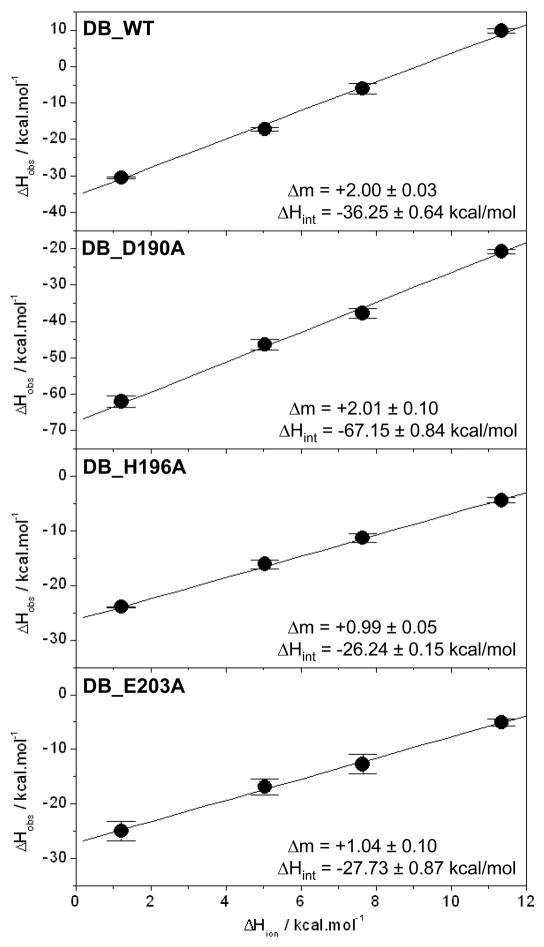
Figure 4.
Dependence of observed enthalpy (ΔHobs) as a function of ionization enthalpy (ΔHion) of various buffers upon the binding of ERE duplex to the wildtype DB domain (DB_WT), the D190A single mutant of DB domain (DB_D190A), the H196A single mutant of DB domain (DB_H196A) and the E203A single mutant of DB domain (DB_E203A) of ERα at pH 7.0 and 25°C. The ΔHion of various buffers used were +1.22 kcal/mol (Phosphate), +5.02 kcal/mol (Hepes), +7.64 kcal/mol (Ticine) and +11.35 kcal/mol (Tris) (33–35). The solid lines within each panel represent fit of data points to Eq [4]. Note that the net change in the number of protons (Δm) absorbed or released per DB monomer upon binding to DNA and the intrinsic binding enthalpy (ΔHint) due to direct protein-DNA interactions and protonation of ionizable moieties for each DB construct are provided within the corresponding panels. Error bars were calculated from 3–4 independent measurements. All errors are given to one standard deviation.