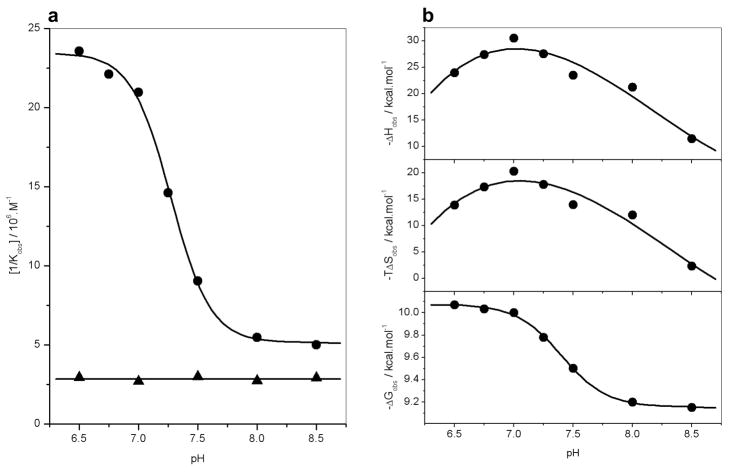
Figure 5.
Dependence of thermodynamics on pH for the binding of ERE duplex to the wildtype DB domain (DB_WT) and the H196A/E203A double mutant of DB domain (DB_AA) of ERα in Phosphate buffer at 25°C. (a) Representative [1/Kobs]-pH plots for the DB_WT (●) and DB_AA (▲) domains. The solid lines respectively indicate sigmoidal and linear fits to data points for clarity. (b) Representative ΔHobs-pH (top panel), TΔSobs-pH (middle panel) and ΔGobs-pH (bottom panel) plots for the DB_WT domain. In the top and middle panels, the solid lines indicate polynomial fits to data points for clarity. In the bottom panel, the solid line indicates sigmoidal fit to data points for clarity.