Table 1.
 | |||||
|---|---|---|---|---|---|
| entry | 1,5-diene | yielda (%) | path selectivityb | stereoselectivity | major productd |
| 1 |
2 |
- | 1.4:1 | 1:1 |
3 |
| 2 |
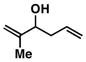 4 |
50 | ≥ 20:1 | - |
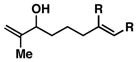 5 |
| 3 |
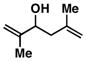 6 |
57 | ≥ 20:1 | ≥ 20:1c |
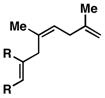 7 |
| 4 |
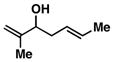 8 |
57 | ≥ 20:1 | ≥ 20:1c |
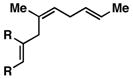 9 |
| 5 |
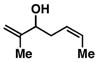 10 |
76 | ≥ 20:1 | ≥ 20:1c |
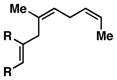 11 |
| 6 |
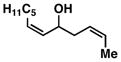 12 |
53e | ≥ 20:1 | ≥ 20:1c |
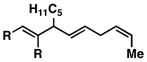 13 |
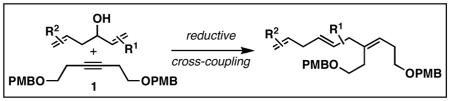
Reaction conditions: 1 (2–3 equiv.), Ti(Oi-Pr)4, c-C5H9MgCl, PhMe (−78 to −35 °C), then cool to −78 °C and add Li alkoxide of the allylic alcohol as a solution in THF (warm to 0 °C).
In cases where selectivity is reported as ≥20:1, no evidence was found for products derived from C–C bond formation by a different path.
In cases where selectivity is reported as ≥ 20:1, no evidence was found for the formation of stereoisomeric products.
Olefin geometry of the major products was assigned by analogy to previous examples.
Yield reported is after HPLC purification.
