FIG. 3.
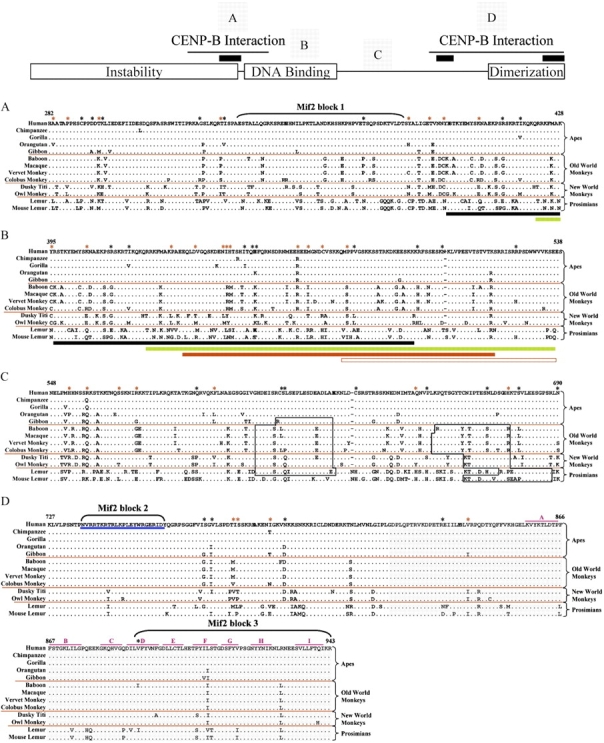
Alignment of the deduced protein sequence of the major functional domains of CENP-C from 13 primates. The major functional domains of the CENP-C protein are indicated in the model at the top. Specifically, the Instability, DNA Binding, and Dimerization domains are shown along with the two CENP-B-Interaction domains (thin black lines) and the three Mif2-homology domains (black bars). The multispecies protein sequence alignment of selected regions (A–D) is shown below the model; the labeled squares above the model show the relative positions of each of these regions. Black asterisks along the top indicate residues under positive selection with posterior probabilities of greater than 0.5; red asterisks reflect residues under positive selection with posterior probabilities of greater than 0.7. Other features of the alignment are as in figure 1. (A) N-Terminal CENP-B-interaction domain (residues 282–428; Suzuki et al. 2004), which overlaps the instability domain (residues 1–373; Lanini and McKeon 1995), contains a Mif2-homology domain (Mif2 block 1, residues 336–383; Brown 1995), and overlaps the start of the DNA-binding domain (residues 395–538; Yang et al. 1996; Sugimoto et al. 1997; Cohen et al. 2008). Black and green bars indicate overlap with the start of the DNA-binding domain (see B). (B) DNA-binding domain (residues 395–538). Highlighted below the alignment are portions of the DNA-binding domain, as determined by previous studies (black bar, residues 396–498 [Sugimoto et al. 1997]; green bar, residues 422–537 [Cohen et al. 2008]; and red bar, residues 433–520 [Yang et al. 1996]). The open red bar indicates the minimal CATD (residues 478–537; Yang et al. 1996). (C) Region containing potential PEST sequences (open black boxes), as determined in this study. (D) C-terminal CENP-B-interaction domain (residues 727–943; Suzuki et al. 2004) and dimerization domain (gray highlighted residues 820–943; Sugimoto et al. 1997), which encompass the other Mif2-homology domains (residues 736–759 and 890–943; Brown 1995), the CENP-C-signature domain (underlined in blue, residues 736–759; Meluh and Koshland 1995), and the 9 (A–I) domains of the β-jelly roll (indicated with pink lines; Dunwell et al. 2001; Cohen et al. 2008).
