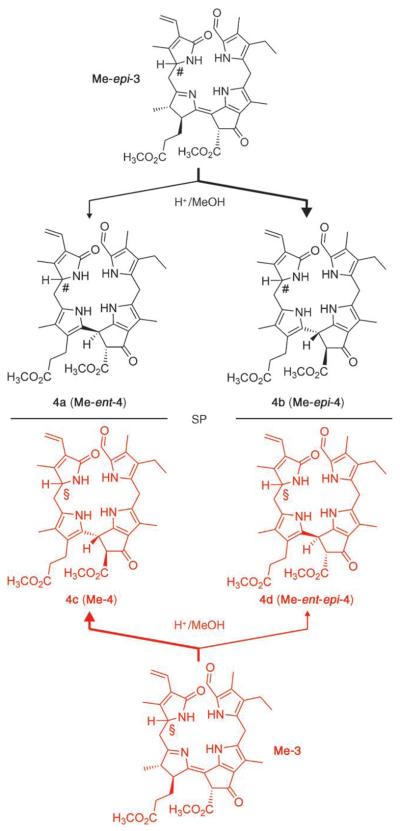
Scheme 3.
Acid-induced isomerization of the FCC methyl esters Me-3 and Me-epi-3 shows reduced stereoselectivity and provides two diastereomeric pairs of NC enantiomers (Me-4/Me-ent-4 and Me-epi-4/Me-ent-epi-4). The identical configuration at C1 in Me-3 and the NCCs 4c and 4d is indicated by §, the opposite common configuration of Me-epi-3 and 4a and 4b by the symbol #. Molecular models support a tentative assignment of S when the configuration at C1 is § and R when the configuration at C1 is #. SP = symmetry plane, bold arrows lead to the major products.