Fig. 2.
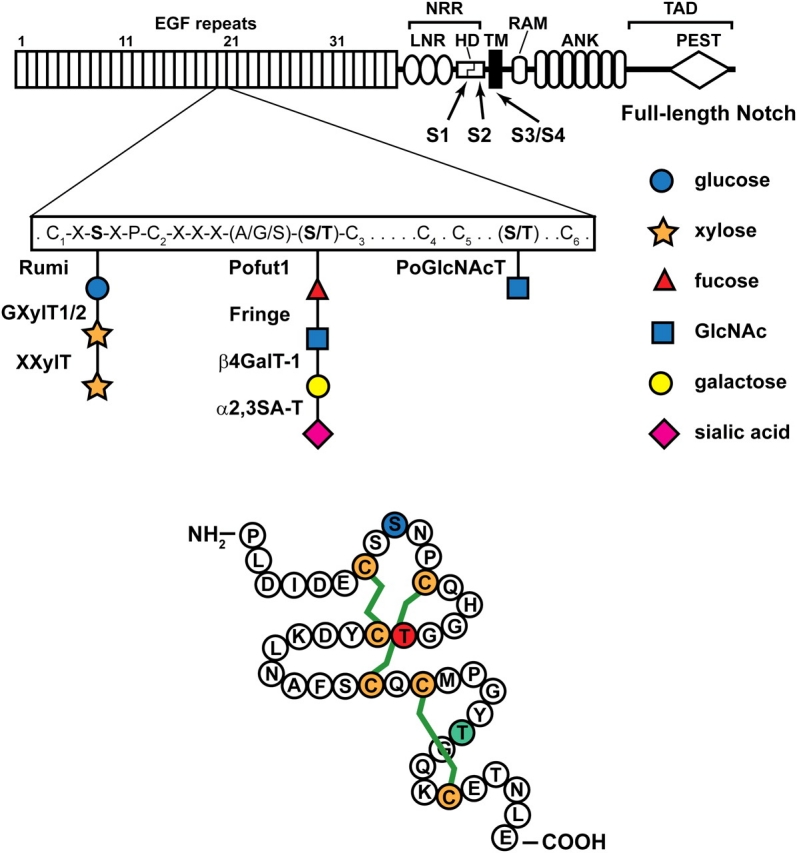
A schematic representation of the full-length Drosophila Notch is shown. The extracellular domain of Notch contains 36 EGF-like repeats, followed by the Negative Regulatory Region (NRR), which consists of three LNR motifs and a heterodimerization domain (HD). The intracellular domain contains an RBP-J association module (RAM), two nuclear localization signals (NLS), seven Ankyrin repeats (ANK) and a PEST domain. The C-terminal part of the intracellular domain contains a trans-activation domain (TAD). Each EGF repeat has six cysteine residues (C1 to C6). The consensus motifs for O-linked glucose and O-linked fucose and the enzymes responsible for their formation and elongation are shown. O-GlcNAc is added to a serine or threonine between C5 and C6, but the exact consensus motif is not known yet. S1–S4 show the position of Notch cleavage sites. TM, transmembrane. The lower drawing is based on the crystal structure of one of the EGF-like repeats of human coagulation factor IX (Huang et al. 1989). The shown amino acid sequence is from EGF-like repeat 20 of the Drosophila Notch, which has experimentally been shown to harbor all three forms of O-linked glycans discussed here (Matsuura et al. 2008). The green lines depict the three disulfide bridges which form between pairs of cysteines in EGF-like repeats (C1–C3, C2–C4, and C5–C6). Blue S, red T and green T show the amino acids to which O-linked glucose, fucose and GlcNAc are attached, respectively
