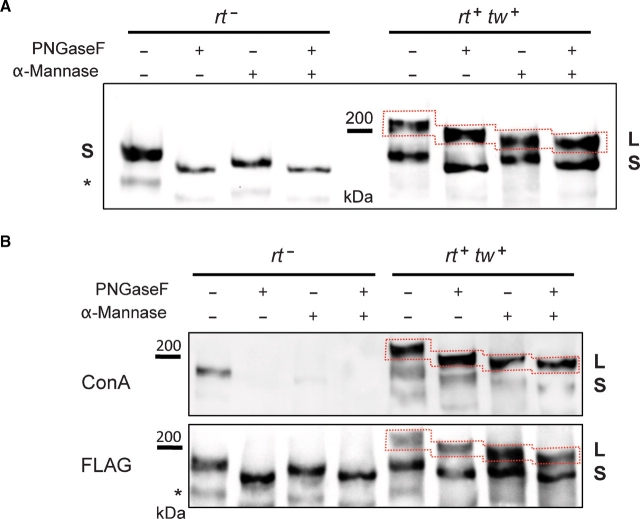
Fig. 4.
Analysis of ExDG glycosylation by glycosidase treatments. (A) left panel: ExDG purified from rt mutants was treated with PNGaseF or α-mannosidase, or with both glycosidases sequentially. No additional shift of the ExDG band (S band) is detected in double PNGaseF/α-mannosidase treatment as compared to PNGaseF treatment alone, suggesting that ExDG has no O-mannose modification. Right panel: glycosidase treatments of ExDG purified from RT-TW coexpression background. Top (L) band shows significant loss of mass (≥10 kDa) when PNGaseF-treated ExDG was digested with α-mannosidase, which suggests the presence of abundant O-mannose modifications. No such loss of mass was detected for the lower (S) band, suggesting that O-mannosylation of ExDG in this band is not significant. (B) Con A reactivity of purified ExDG after treatments with PNGaseF and α-mannosidase. The S glycoform purified from rt mutant background loses Con A reactivity either after the removal of N-linked glycans by PNGaseF or after treatment with α-mannosidase removing α-linked mannose residues (top panel, left), suggesting the absence of O-mannose modification and efficient removal of oligomannose structures either by trimming N-linked branches with α-mannosidase or by complete elimination of N-linked glycans. At the same time, the L glycoform purified from RT-TW co-expression background retains Con A reactivity after treatment with PNGaseF, α-mannosidase, or both glycosidases (top panel, right), suggesting that L glycoform is O-mannosylated, and that α-mannosidase does not remove O-mannose completely. The bottom panel shows anti-FLAG western control corresponding to the lectin blot. Red dashed line outlines the region of the L glycoform on the blots. Asterisk “*” indicates an additional minor band sometimes detected by FLAG Western blots that probably represents ExDG proteolytic degradation.