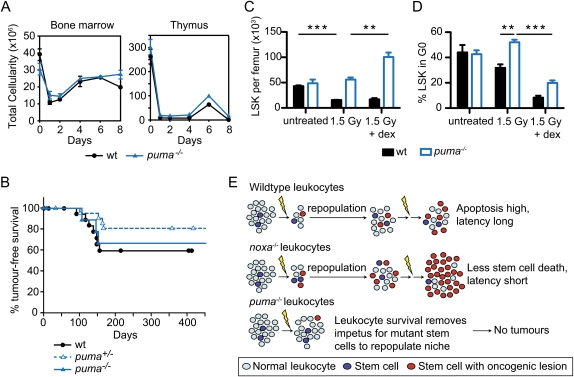
Figure 4.
Leukocyte depletion by dexamethasone restores γ-irradiation-induced thymic lymphoma development in Puma-deficient mice. (A) Total cellularity of the bone marrow and thymus of wild-type and puma−/− mice after combined treatment with γ-irradiation (1.5 Gy) plus dexamethasone (250 μg) on days 0 and 7. Mean ± SEM, n = 2. (B) Kaplan-Meier curves showing percentage tumor-free survival of mice after four weekly combined treatments with γ-irradiation (1.5 Gy) and dexamethasone. For wild-type mice, n = 21; for puma+/− mice, n = 22; and for puma−/− mice, n = 10 (P > 0.35 for all comparisons). (C) Impact of combined treatment with γ-irradiation and dexamethasone on numbers of LSK cells in wild-type and puma−/− mice. LSK cells were analyzed 3 d following treatment with γ-irradiation (1.5 Gy) or γ-irradiation (1.5 Gy) plus dexamethasone (250 μg). Mean ± SEM, n = 4, (**) P < 0.01; (***) P < 0.001. (D) Cell cycle status of LSK cells from mice treated as in C. Mean ± SEM, n = 3. Supplemental Figures 8 and 9 provide FACS data of the cell cycle analysis. (E) Model of thymic lymphoma development in Noxa- and Puma-deficient mice (see the text). We propose that, in wild-type mice, the γ-irradiation both creates oncogenic lesions within rare cells of the hematopoietic stem/progenitor cell compartment and, by killing most differentiated leukocytes, recruits those cells into the cell cycle to repopulate the compartment. In Noxa-deficient mice, the differentiated leukocytes die normally, but more of the stem/progenitor cells bearing potentially oncogenic mutations survive, thereby hastening tumorigenesis. In Puma-deficient mice, however, the survival of most differentiated cells removes the impetus for recruitment of the mutant stem/progenitor cells that would otherwise found a tumor.