Fig. 1. ER chaperones associated with purified HLA-B27 heavy chains.
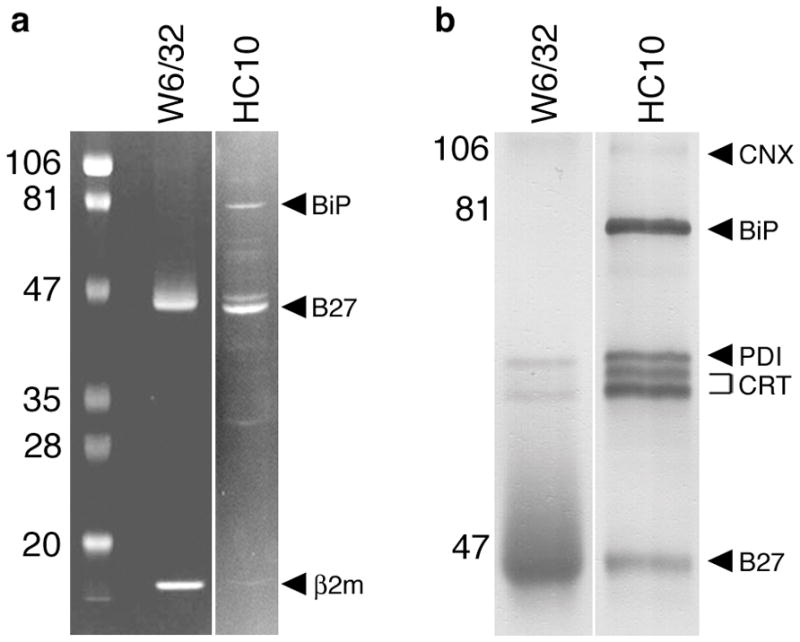
HLA-B27 was purified from C1R.B27 cell lysates using sequential W6/32 and HC10 immunoaffinity columns. (A) Coomasie blue stain of HLA-B27 purified on W6/32 and HC10 columns separated on SDS-PAGE under reducing conditions. A reverse image is shown to enhance visualization of faint bands. HLA-B27, β2m, and BiP are indicated by arrows (right) and pre-stained markers (left) by their MW. BiP was identified by mass spectroscopic (MALDI-TOF) analysis of in-gel digested protein (data not shown). (B) Immunoblots of purified HLA-B27 performed using a cocktail of antibodies including AF-8 (anti-calnexin; CNX), anti-BiP (BiP), anti-PDI (PDI), anti-calreticulin (CRT), and 3B10.7 (anti-class I HC; B27). Prior to preparing the cocktail chaperones were first identified by immunoblotting with single antibodies (not shown). Calreticulin migrates as two forms indicated by the bracket. Band intensity in (A) reflects relative amount of protein stained with Coomassie blue.
