Fig. 2. HLA-B27 misfolding in the endoplasmic reticulum and dimers on the cell surface.
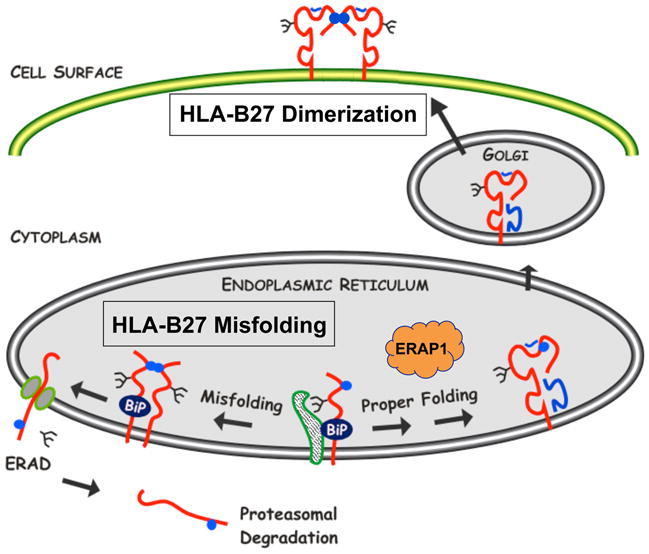
Newly synthesized HLA-B27 heavy chains exhibit three features of protein misfolding: (i) prolonged and stable association with the ER chaperone BiP, (ii) formation of disulfide-linked dimers and possibly oligomers, and (iii) enhanced ERAD. ERAD involves dislocation of heavy chains from the lumen of the ER into the cytosol via the Sec61 complex and involves heavy chain deglycosylation and proteasomal degradation. HLA-B27 also has a tendency to form homodimers via endosomal recycling that can be expressed on the cell surface. Cell surface dimers shown here are in a folded conformation. Unfolded dimers as well as unfolded monomers also exist on the cell surface in varying amounts. Heavy chains are shown in red, with β2m and peptide in blue. Blue circles depict the unpaired Cys67 residue. Other Cys residues have also been implicated in aberrant disulfide bond formation. The figure is reproduced in a modified form from ref. (7) with permission.
