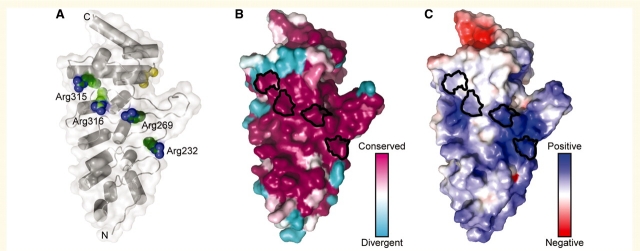
Figure 3.
The four mutations at arginine residues in the TRPV4 ARD localize to the same conserved, positively charged protein surface. (A) A ribbon representation of the structure of the TRPV4 ARD from chicken (Landoure et al., 2010), with a transparent molecular surface. The side chains of Arg232, Arg269 and Arg315 (Arg218, Arg255 and Arg301, respectively, in chicken) are shown in green ‘van der Waals’ sphere representation, and the previously identified Arg316 (Arg302 in chicken) (Deng et al., 2010) is shown in light green. The backbone positions of the skeletal dysplasia mutations at Ile331 and Asp333 (Krakow et al., 2009) are shown as yellow spheres for reference. (B) The sequence conservation in TRPV4, using an alignment of 27 TRPV4 orthologues, is mapped onto the surface of the structure. Note that Arg232, Arg269, Arg315 and Arg316 are strictly conserved as arginine residues in available TRPV4 orthologues. (C) The solvent-accessible electrostatic properties of the protein are mapped onto the molecular surface, with blue representing positively charged surfaces (+5 kT) and red, negatively charged surfaces (–5 kT). In (B) and (C) the surface regions corresponding to Arg232, Arg269, Arg315 and Arg316 are outlined in black.