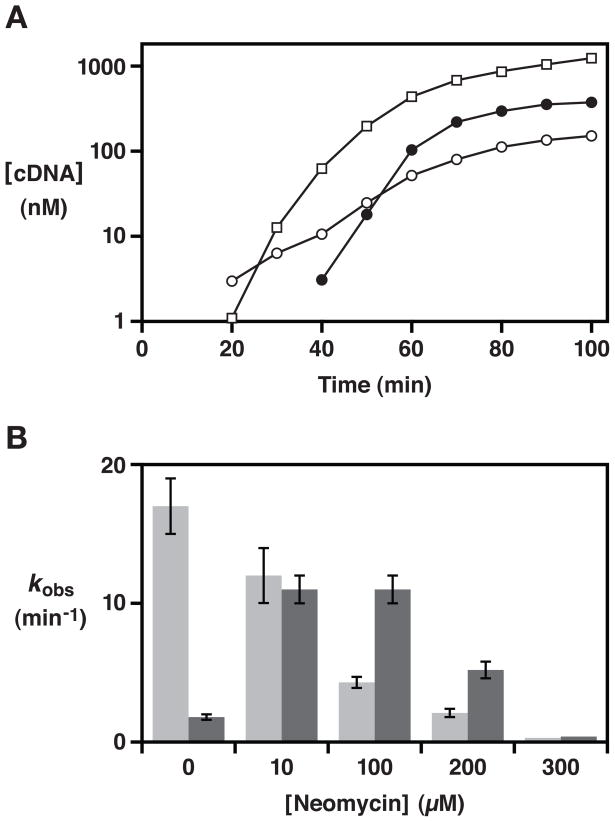
Figure 3.
Biochemical properties of the wild-type and neomycin-resistant forms of the class I ligase. (A) Amplification profiles comparing the wild-type enzyme in the absence of neomycin (open squares) and the evolved enzyme in either the absence (open circles) or presence (filled circles) of 100 μM neomycin. Amplification was initiated with 1 nM RNA enzyme and 2.5 μM substrate, and the yield of full-length cDNA was determined at various times. No amplification of the wild-type enzyme was observed in the presence of 100 μM neomycin. (B) Catalytic activity of the wild-type (light gray) and evolved (dark gray) enzymes in the presence of various concentrations of neomycin. Reaction conditions: 30 nM enzyme, 2.5 μM substrate, 10 mM MgCl2, and 50 mM KCl at pH 7.5 and 37 °C.