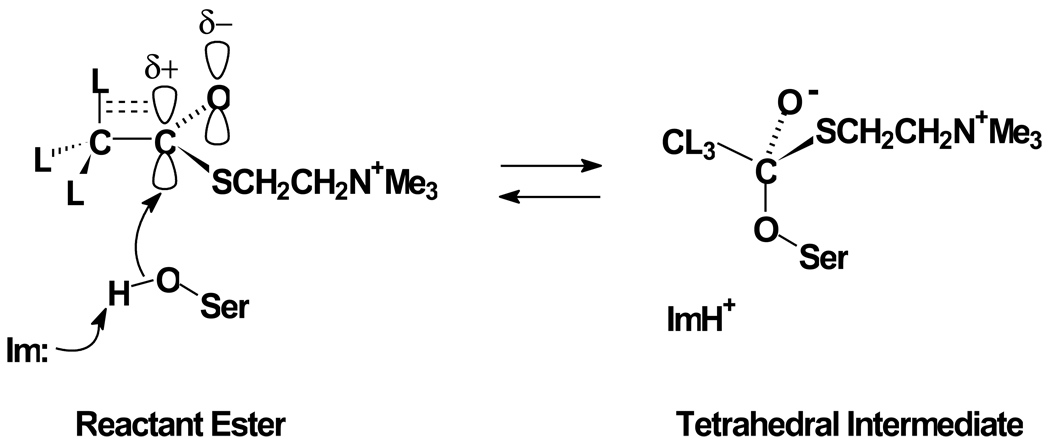
Fig. 1.
Structural origins of β-secondary deuterium isotope effects for the formation of the tetrahedral intermediate in the acylation stage BuChE-catalyzed hydrolysis of ATCh. The γ-oxygen of the active site serine covalently adds to the carbonyl carbon of the thioester function of the substrate, with general-base assistance by the imidazole sidechain (Im) of the active site histidine. This nucleophilic addition is marked by change of the hybridization of the carbonyl carbon from sp2 in the reactant state to sp3 in the tetrahedral intermediate.