Abstract
Desmin and vimentin are intermediate filaments that play crucial roles in the maturation, maintenance and recovery of muscle fibers and mesenchymal cells. The expression of these proteins has not been investigated extensively in human fetuses. In the present study, we examined the immunohistochemical expression of intermediate filaments in skeletal muscles of the head, neck and thorax in 12 mid-term human fetuses at 9–18 weeks of gestation. We also used immunohistochemistry to localize the expression of the myosin heavy chain and silver impregnation to identify the fetal endomysium. Expression of desmin and vimentin was already detectable in intercostal muscle at 9 weeks, especially at sites of muscle attachment to the perichondrium. At this stage, myosin heavy chain was expressed throughout the muscle fibers and the endomysium had already developed. Beginning with punctate expression, the positive areas became diffusely distributed in the muscle fibers. At 15–18 weeks, intermediate filament proteins were extensively expressed in all of the muscles examined. Expression at the bone–muscle interface was continuous with expression along the intramuscular tendon fibres. These results suggest that the development of intermediate filaments begins in areas of mechanical stress due to early muscle contraction. Their initially punctate distribution, as observed here, probably corresponds to the earliest stage of fetal enthesis formation.
Keywords: desmin, enthesis, human fetus, intercostal muscle, vimentin
Introduction
Desmin, a major intermediate filament component, plays a crucial role in the maturation, maintenance and recovery of skeletal and cardiac muscle fibers because it forms an interlinking scaffold around myofibrils with connections to the sarcolemma and nuclear membrane (Tidball, 1992; Goldfarb et al. 2008). In normal adult skeletal muscle, desmin is abundant at myotendinous and neuromuscular junctions (Carlsson et al. 1999). Vimentin, another type of intermediate filament, coexists with desmin in human fetal skeletal muscle fibers and plays an important role in maintaining the stability of mesenchymal cells and also in signal transduction (Yang & Makita, 1996). Vimentin is also known to be a marker of osteoprogenitor cells (Watanabe et al. 1993; Shapiro et al. 1995; Xian et al. 2004) and is expressed in the cell body and processes of osteoblasts and osteocytes (Shapiro et al. 1995).
The development of these intermediate filaments is probably closely related to muscle fiber maturation. However, to our knowledge, their expression has not been fully examined in human fetal muscles. In particular, there is no information to indicate whether the expression of desmin and vimentin exhibits heterogeneity at a specific site in a muscle (e.g. the center or periphery of a muscle fiber), a region of the body (e.g. the head, thorax or upper extremity) or a specific developmental stage. The present immunohistochemical study was therefore conducted to investigate the expression and distribution of these intermediate filaments in human fetal skeletal muscles. For easy understanding of the intramuscular distribution of positive reactivity, we used relatively large sections showing the topographical anatomy of the muscles examined.
Materials and methods
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We examined the skeletal muscle of the head, neck and thorax in 12 mid-term human fetuses at 9–18 weeks of gestation (three fetuses in each group). With the agreement of the families concerned, these specimens were donated to the Department of Anatomy, Chonbuk National University in Korea. Moreover, their use for research was approved by the university ethics committee and this study was performed in accordance with the rules of the facility to which the lead author belongs. The fetuses had been obtained by induced abortions. After abortion, each of the mothers was personally informed by an obstetrician about the possibility of donating the fetus for research; no attempt was made to encourage donation. The donated fetuses were fixed with 10% w/w formalin solution for more than 3 months. Because of randomization of the specimen numbering, it was not possible to trace any of the families concerned.
Immunohistochemical procedures
In order to analyze the expression of protein in the samples, their immunolocalization of desmin, vimentin and myosin was investigated. The primary antibodies used were: (i) mouse monoclonal anti-human striated muscle myosin heavy chain (dilution, 1 : 100; Dako, Glostrup, Denmark); (ii) mouse monoclonal anti-human vimentin (dilution, 1 : 10; Dako); and (iii) mouse monoclonal anti-human desmin (dilution, 1 : 50; Dako). Pretreatment with an autoclave was not conducted because of the fragile nature of the fetal tissues. The secondary antibody (Chem Mate Envison kit; Dako) was labeled with horseradish peroxidase and antigen–antibody reactions were detected via an horseradish peroxidase-catalyzed reaction with diaminobenzidine. Counterstaining with hematoxylin was performed on the same samples.
In addition, silver impregnation staining (Lillie et al. 1980) was performed to show the endomysium, in which argyrophilic reticular fibers were stained black and collagen fibers were stained wine red.
Results
Immunohistochemical examination
Expression of desmin
High immunoreactivity for desmin was seen mostly in the head, neck, and thoracic and shoulder girdle muscles at 9–12 weeks. In the intercostal muscles, a site of desmin expression was clearly localized at the rib insertion, with a weak or negative reaction in the central part of the muscles (Fig. 1). The specimens did not show evidence of the diaphragm at 9 weeks. However, at 12 weeks, positive immunoreaction of desmin was diffusely expressed in the diaphragm. At 12 weeks, desmin immunolocalization was identifiable in the masticatory, tongue, and shoulder girdle and back muscles (Fig. 2). The spots of immunoreactivity were distributed along intramuscular aponeurotic tissues (or intramuscular tendons) as well as at bone or cartilage insertions. The intermediate tendon of the omohyoideus also showed sites of desmin expression. In the tongue, sites of desmin expression appeared near the sagittal septum (Fig. 2A). Among the complex muscles of facial expression, sites of desmin expression were found at the insertion to the base of the nasal cartilages.
Fig. 1.
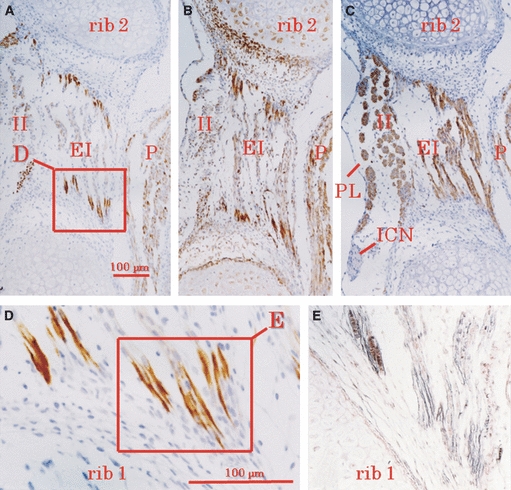
Immunohistochemistry of human fetal intercostal muscles at 9 weeks. (A, B) Immunoreactivity for desmin and vimentin is restricted to the muscle insertions. (C) Myosin heavy chain is distributed diffusely throughout the muscles. (D) Higher magnification view of the square in A. (E) Silver impregnation demonstrates the endomysium (stained black) connecting to the perichondrium of the rib (stained wine red). EI, external intercostal muscle; II, internal intercostal muscle; ICN, intercostal nerve; P, pectoralis major (caudal end); PL, pleural cavity; rib 1 and rib 2, first and second ribs.
Fig. 2.
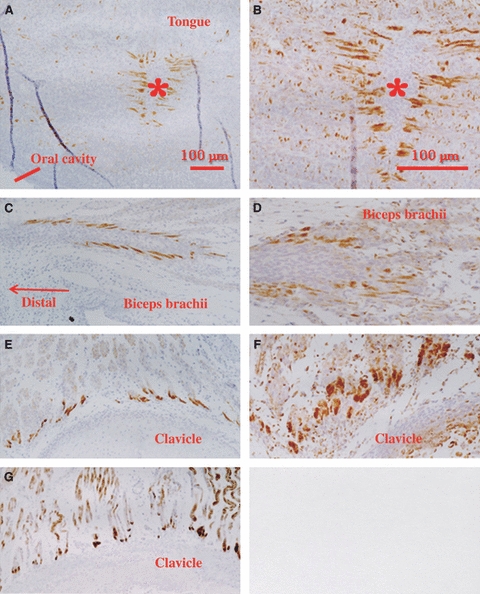
Condensations of desmin and vimentin in 12-week human fetus. (A, C, E, G) Desmin immunohistochemistry. (B, D, F) Vimentin expression. (A, B) In the tongue, condensations (spots) of desmin or vimentin expression are evident near the sagittal septum (asterisk). (C, D) In the biceps brachii muscle, the spots are arranged in line along the distal tendon. (E–G) In the trapezius muscle, the spots are located along the insertion into the clavicle (E, F). (G) The trapezius muscle fibers exhibit desmin spots in spite of their diffuse desmin expression at 15 weeks.
At 15 weeks, because the entire length of muscle fibers had become diffusely positive for desmin, sites of desmin expression became indistinct in some muscles, including the intercostal muscles (Figs 2G, 3 and 4). Sites of desmin expression then became evident in muscles containing thick intramuscular tendons (e.g. the muscles around the shoulder joint; Fig. 3). It was notable that sites of desmin expression were not clearly identified near the sagittal septum of the tongue due to the diffuse nature of the desmin expression, and instead became distinctive around the parasagittal septum (Fig. 4A). At 18 weeks, in most muscles including the intercostals, the punctate expression of desmin changed into diffuse expression throughout the entire muscle. In back muscles extending between the vertebral transverse processes at 15–18 weeks, the appearance of sites of desmin expression was delayed, even though the muscle configuration was similar to that of the intercostals. Moreover, in some muscles, such as the serratus anterior and multifidus, desmin localization was evident not only at the muscle insertion but also in the central part of the muscle fibers.
Fig. 3.
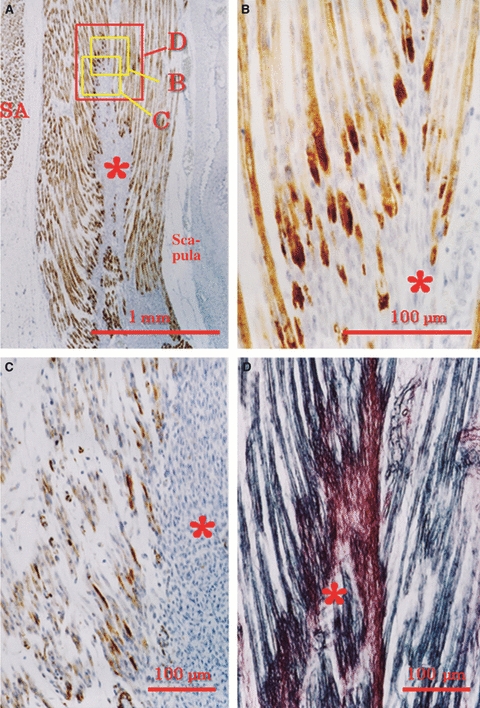
Subscapularis muscle in a human fetus at 15 weeks. (A, B) Immunohistochemistry for desmin (B, higher magnification). (C) Immunohistochemistry for vimentin in an adjacent section. (D) Silver impregnation of the section adjacent to that in A. Orientations of B–D are shown as squares in A. The desmin or vimentin spots (B and C) are evident in and near the intramuscular tendon (asterisk). (D) The black-stained endomysium connects to the wine red-stained intramuscular tendon. SA, serratus anterior.
Fig. 4.
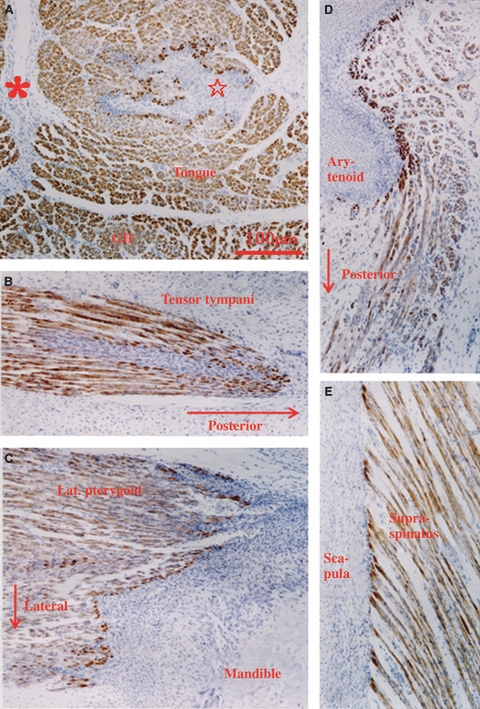
Sites of desmin expression in muscles of a human fetus at 15 weeks (a different specimen from that shown in Fig. 3). (A) Sites of desmin expression are not evident along the sagittal septum of the tongue (asterisk) but are evident around the parasagittal septum (star). GH, geniohyoideus. (B) Tendon of the tensor tympani with sites of desmin expression. (C) Lateral pterygoid muscle inserts to the mandibular head. (D) Laryngeal muscle inserting to the posterolateral part of the arytenoid cartilage. (E) Origin of the supraspinatus muscle from the scapular plate. All figures were prepared at the same magnification.
Expression of vimentin
Sites of desmin expression, localization within a muscle, and the chronological sequence of expression were similar to those observed for desmin immunoreactivity. At 9–12 weeks, sites of vimentin expression were evident at the rib insertions of intercostal muscles (Fig. 1B) but the expression became diffusely positive at 15 weeks in these muscles. Notably, at 15 weeks, sites of vimentin expression were extensively distributed along intramuscular tendons and at the muscle–tendon interface (Figs 2B,D,F and 3C), being apparently more frequent than desmin expression. At 18 weeks, vimentin was diffusely expressed throughout the muscle fibers. In addition, osteoprogenitor cells strongly expressed vimentin along the margins of ossification sites. The dura mater and membranous inner ear were also positive for vimentin.
Expression of the myosin heavy chain
The myosin heavy chain is abundantly present in fetal skeletal muscle. Accordingly, all samples showed diffuse expression in the muscle fibers at 9 weeks (Fig. 1C) and we were unable to determine the developmental stage at which myosin expression was initiated.
Silver staining
The spots were located diffusely within the end of the muscle fibers according to silver staining and the nuclei were likely to overlap with the reactive zone (Fig. 1E).The endomysium was stained black by silver impregnation (Figs 1E and 3D) and collagen bundles in the perichondrium and tendon were stained wine red. In the intercostal muscles, the endomysium had already developed by 9 weeks. Likewise, the endomysium was evident when desmin displayed expression at the sites of muscle fiber attachment.
Discussion
The present study appears to be the first to have demonstrated the chronological sequence and heterogeneous distribution of desmin expression in human fetuses. Desmin expression was apparently positive in the intercostal muscles and thereafter seemed to spread to other muscles. The early appearance of desmin expression in the intercostal muscles was probably attributable to the short muscle fibers there, as tension is conducted easily along the entire length of such short fibers in comparison with long fibers. Even in comparison with small muscles of adults, such as the tensor tympani and subclavius, the muscle fibers in fetuses seemed to be larger than in intercostal muscles. Although the intercostal and intervertebral muscles are morphologically similar, the difference in the developmental stage at which desmin expression first appeared in them might be explained by a difference in the stage at which they begin contraction.
Desmin expression in muscle attachments to the pericondrium (or at future sites of bone insertion) was followed by expression along the intramuscular tendons. In lingual muscles, desmin expression was initiated from the midsagittal septum and then the site of strongest expression moved to the parasagittal septum. The expression of vimentin also demonstrated a similar chronological sequence. The results from week 15 would suggest that the expression occurs in muscle cells (Hesselmans et al. 1993). Such heterogeneity in the site and stage of expression suggested progressive maturation of the muscle fiber attachments. Expression of the myosin heavy chain (Gojo et al. 2002; Usami et al. 2003; Maejima et al. 2005; Shida et al. 2005; Okubo et al. 2006; Yanagisawa et al. 2006; Abe et al. 2007; Suzuki et al. 2007; Yoshii et al. 2008) appeared to have been completed before that of desmin was initiated. Likewise, silver impregnation showed that the endomysium of each muscle fiber was apparently in tight contact with the perichondrium, insertion tendon or intramuscular tendon until the expression of desmin appeared. Therefore, it was suggested that desmin expression coincided with the initiation of mechanical stress between the endomysium and mature muscle fiber accompanying early contraction.
Vimentin, another intermediate filament, is a well-known marker of osteoprogenitor cells (Watanabe et al. 1993; Shapiro et al. 1995; Xian et al. 2004) and plays an important role in maintaining the stability of mesenchymal cells (Yang & Makita, 1996). Thus, the concomitant expression of both vimentin and desmin observed in this study appeared to suggest maturation of the developing muscle–tendon and muscle–bone interfaces. During the development of rabbit fetal limb muscles, insertion regions are characterized by the presence of coarse-fibered periosteal bone and chondroid bone, both being morphologically intermediate between fibrocartilage and lamellar bone (Hurov, 1986). Fibrocartilage provides the bone–tendon interface or enthesis; the fibrocartilage cells are commonly packed with intermediate filaments, which could be involved in the transduction of mechanical load (Benjamin et al. 2006). Although previous studies did not include any data for early stages of development, the punctate expression of desmin and vimentin observed in the present study probably corresponded to the morphology present at the earliest stage of enthesis development.
The present findings were consistent with those of a previous study of human fetal cardiac muscles, in which desmin expression increased progressively with fetal age (Kim, 1996). Notably, that study also demonstrated that, in contrast to desmin, vimentin-positive areas diminished progressively in cardiac muscle, despite the fact that vimentin is present not only in muscle cells but also connective tissue cells. A similar dynamic relationship between desmin and vimentin has been reported in human skeletal muscle fibers (Barbet et al. 1991; Yang & Makita, 1996). In the present study, vimentin immunoreactivity extended diffusely throughout muscle fibers by 18 weeks. However, this observation did not indicate a decrease of expression. Unfortunately, we were unable to obtain any data for stages later than 18 weeks because of the limited materials available.
It is well known that the periosteum plays an essential role in tendon and ligament migration during the growth of long bones (Muhl & Gedak, 1986). However, osteoblasts are likely to control the activity of osteoclasts, as well as the distribution of differentiated osteoclasts, via the RANK-RANKL molecular system (Takahashi et al. 1999; Yamamoto et al. 2006). Further studies may reveal a type of osteoblast differentiation in the periosteum that is regulated by a molecular cascade during muscle fiber development, maturation and degeneration.
Acknowledgments
This research was supported by Oral Health Science Center Grant hrc8(Shinichi Abe) from Tokyo Dental College, and by a “Project for Private Universities: matching fund subsidy” from MEXT (Ministry of Education, Culture, Sports, Science and Technology) of Japan, 2010–2012.
References
- Abe S, Sakiyama K, Ide Y. Muscle plasticity: changes in oral function of muscle fiber characteristics. J Oral Biosci. 2007;49:219–223. [Google Scholar]
- Barbet JP, Thornell LE, Butler-Browne GS. Immunocytochemical characterisation of two generations of fibers during the development of the human quadriceps muscle. Mech Dev. 1991;35:3–11. doi: 10.1016/0925-4773(91)90036-6. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Toumi H, Ralphs JR, et al. Where tendons and ligaments meet bone: attachment sites (entheses) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson L, Li Z, Paulin D, et al. Nestin is expressed during development and in myotendinous and neuromuscular junctions in wild type and desmin knock-out mice. Exp Cell Res. 1999;251:213–223. doi: 10.1006/excr.1999.4569. [DOI] [PubMed] [Google Scholar]
- Gojo K, Abe S, Ide Y. Characteristics of myofibers in the masseter muscle of mice during postnatal growth. Anat Histol Embryol. 2002;31:105–112. doi: 10.1046/j.1439-0264.2002.00368.x. [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Olivé M, Vicart P, et al. Intermediate filament diseases: desminopathy. Adv Exp Med Biol. 2008;642:131–164. doi: 10.1007/978-0-387-84847-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmans LF, Jennekens FG, Van den Oord CJ, et al. Development of innervation of skeletal muscle fibers in man: relation to acetylcholine receptors. Anat Rec. 1993;236:553–562. doi: 10.1002/ar.1092360315. [DOI] [PubMed] [Google Scholar]
- Hurov JR. Soft-tissue bone interface: how do attachments of muscles, tendons, and ligaments change during growth? A light microscopic study. J Morphol. 1986;189:313–325. doi: 10.1002/jmor.1051890309. [DOI] [PubMed] [Google Scholar]
- Kim HD. Expression of intermediate filament desmin and vimentin in the human fetal heart. Anat Rec. 1996;246:271–278. doi: 10.1002/(SICI)1097-0185(199610)246:2<271::AID-AR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Lillie RD, Tracy RE, Pizzolato P, et al. Differential staining of collagen types in paraffin sections: a color change in degraded forms. Virchows Arch A Pathol Anat Histol. 1980;386:153–159. doi: 10.1007/BF00427227. [DOI] [PubMed] [Google Scholar]
- Maejima M, Abe S, Sakiyama K, et al. Changes in tongue muscle fiber properties of mouse before and after weaning. Arch Oral Biol. 2005;50:988–993. doi: 10.1016/j.archoralbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Muhl ZF, Gedak GK. The influence of periosteum on tendon and ligament migration. J Anat. 1986;145:161–171. [PMC free article] [PubMed] [Google Scholar]
- Okubo K, Abe S, Usami A, et al. Changes in muscle-fiber properties of the murine digastric muscle before and after weaning. Zool Sci. 2006;23:1079–1084. doi: 10.2108/zsj.23.1079. [DOI] [PubMed] [Google Scholar]
- Shapiro F, Cahill C, Malatantis G, et al. Transmission electron microscopic demonstration of vimentin in rat osteoblast and osteocyte cell bodies and processes using the immunogold technique. Anat Rec. 1995;241:39–48. doi: 10.1002/ar.1092410107. [DOI] [PubMed] [Google Scholar]
- Shida T, Abe S, Sakiyama K, et al. Superficial and deep layer muscle-fiber properties of the mouse masseter before and after weaning. Arch Oral Biol. 2005;50:65–71. doi: 10.1016/j.archoralbio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Abe S, Kim H, et al. Changes in the muscle fibre properties of the mouse temporal muscle after weaning. Anat Histol Embryol. 2007;36:103–106. doi: 10.1111/j.1439-0264.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Udagawa N, Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun. 1999;256:449–455. doi: 10.1006/bbrc.1999.0252. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Desmin at myotendinous junctions. Exp Cell Res. 1992;199:206–212. doi: 10.1016/0014-4827(92)90425-8. [DOI] [PubMed] [Google Scholar]
- Usami A, Abe S, Ide Y. Myosin heavy chain isoforms of the murine masseter muscle during pre and postnatal development. Anat Histol Embryol. 2003;32:244–248. doi: 10.1046/j.1439-0264.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Miake K, Sasaki J. Immunohistochemical study of the cytoskeleton of osteoblasts in the rat calvaria. Intermediate filaments and microfilaments as demonstrated by detergent perfusion. Acta Anat. 1993;147:14–23. [PubMed] [Google Scholar]
- Xian CJ, Zhou FH, McCarty RC, et al. Intramembranous ossification mechanism for bone bridge formation at the growth plate cartilage injury site. J Orthop Res. 2004;22:417–726. doi: 10.1016/j.orthres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Udagawa N, Matsuura S, et al. Osteoblasts provide a suitable microenvironment for the action of receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2006;147:3366–3374. doi: 10.1210/en.2006-0216. [DOI] [PubMed] [Google Scholar]
- Yanagisawa N, Abe S, Agematsu H, et al. Myosin heavy chain composition of tongue muscle in microphthalmic (mi/mi) mice before and after weaning. Ann Anat. 2006;188:329–336. doi: 10.1016/j.aanat.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Yang Y, Makita T. Immunocytochemical colocalization of desmin and vimentin in human fetal skeletal muscle cells. Anat Rec. 1996;246:64–70. doi: 10.1002/(SICI)1097-0185(199609)246:1<64::AID-AR7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Yoshii M, Sakiyama K, Abe S, et al. Changes in the myosin heavy chain 2a and 2b isoforms of the anterior belly of the digastric muscle before and after weaning in mice. Anat Histol Embryol. 2008;37:147–152. doi: 10.1111/j.1439-0264.2007.00813.x. [DOI] [PubMed] [Google Scholar]


