Abstract
Objective
The present investigation tested the hypothesis that intrinsic gender-related differences exist in rat aortic smooth muscle cell MMP2.
Methods
This investigation comprised three sets of experiments. Experiment I: Adult male and female rat aortic smooth muscle cells (RASMCs) at passages 4–8 were stimulated in serum-free media for 48 hours with IL1β at doses encountered in human AAAs (2ng/mL). Messenger RNA was extracted from the RASMCs, and gene expression of MMP2 and tissue inhibitor of metalloproteinase2 (TIMP2),a major MMP2 inhibitor, was measured by real-time polymerase chain reaction. MMP2 protein levels in conditioned media were measured by Western Blotting, and MMP2 and TIMP2 activity quantified by standard and reverse gelatin zymography. Experiment II: Male and female RASMCs were incubated for 48 hrs in DMEM containing IL-1β and 17-β-estradiol at doses from 1×10−10 to 1×10−6 molar. MMP2 activity in the conditioned media was then determined. Experiment III: Male rats underwent sustained 17-β-estradiol exposure for 21 days using extended-release, subcutaneously implanted pellets prior to sacrifice and aortic explantation. Aortas from males, females, and estradiol-treated males were stimulated with IL1β for 48 hrs, and MMP2 activity in the conditioned media was determined.
Results
Experiment I: MMP2 gene expression was 3-fold higher in male compared to female IL1β stimulated RASMCs (P<0.0001). MMP2: TIMP2 gene expression ratio was 7.5 fold greater in male vs. female RASMCs. MMP2 protein levels were 3-fold higher (2.68 vs. 0.96 O.D./mg total protein, P=0.003) in male vs. female RASMCs. Gelatinolytic activity was more than 6-fold higher (15,010 vs. 2,472 O.D./mg total protein, P=0.002) in male vs. female RASMCs. Experiment II: MMP2 activity in male and female RASMCs was not altered by a wide range of 17-β-estradiol concentrations. Experiment III: When pre-treated with 17-β-estradiol, MMP2 activity in the media of male rat whole-aortic explants decreased two-fold ( P=0.002). This post-17-β-estradiol treatment male level was not different than baseline female aortic explant MMP2 levels.
Conclusions
MMP2 is higher in male RASMCs compared to female RASMCs. Exogenous 17-β-estradiol did not alter MMP2 activity in vitro, but in vivo 17-β-estradiol exposure greatly decreased male aortic MMP2 production to levels seen in the female aorta. Gender differences in MMP2 are speculated to be associated with phenotypic differences in human AAA formation.
Background
Matrix metalloproteinases are a family of proteinases that are strongly associated with abdominal aortic aneurysms (AAAs). Specifically, matrix metalloproteinase2 (MMP2; Gelatinase A; 72kDa Gelatinase) is produced by vascular smooth muscle cells[1] and is increased in both human[2–4] and experimental murine AAAs[5]. MMP2 appears critical in the formation of experimental murine AAAs[5]; it has been shown that mice deficient in MMP2 are highly resistant to experimental aneurysm formation.[6] Additionally, inhibitors of MMP2 have been shown to decrease aortic wall elastin degradation.[7]
In addition to the long-standing[8] and repeated association of MMP2 and abdominal aortic aneurysm disease, it has long been observed that the incidence and size of human AAAs are greater in males than females,[9, 10] but that the differences tend to decrease in post-menopausal women. While the basis of the gender-related disparity is not known, the same phenomenon is observed in experimental animal models of AAA[11]. Given the association of MMP2 and AAA formation, and the observation of gender-related differences in AAA, this investigation tested the hypothesis that intrinsic gender-related differences exist in aortic smooth muscle cell MMP2. Additionally, we sought to determine if modifications to the local hormone environment would alter MMP2 activity.
Methods
Cell Culture
Reagents were obtained from Sigma Chemical Co. (St. Louis, MO) unless otherwise indicated. All experiments were performed with approval of the University of Michigan Committee on Laboratory Animal Medicine. RASMCs were cultured from the abdominal aortas of young (190–210 gm) male and female Sprague-Dawley rats (Charles River Labs, MA). After animal sacrifice and aortic explantation under general inhalational anesthesia, the aortic tissue was cut into 2 mm2 pieces and placed in 60 mm diameter plastic tissue culture dishes. Basement membrane Matrigel™ (Collaborative Research, Bedford, MA) was applied to each section of explanted tissue to prevent floating. Cultures were grown in Dulbecco's modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) (HyClone Laboratories, Logan, UT), 100 units/mL penicillin, 100 µg/mL streptomycin, and glutamine 292 mcg/mL. Tissue culture media and antibiotics were obtained from Gibco (Rockville, MD). Tissues were incubated at 37°C in a humidified, 5% CO2 atmosphere for 4 to 7 days, until spindle-shaped SMC were observed extending from the tissue. After removing the explant, cells were dispersed by treatment with trypsin (Gibco), centrifuged, resuspended in complete medium, and placed into 75 cm2 culture flasks. Post-confluent cultures assumed a hill and valley topography characteristic of SMCs grown in vitro. RASMCs were confirmed by staining with a monoclonal antibody against SMC-specific α-actin and determined to nearly 100% SMCs.
Experiment I
Adult male and female rat aortic smooth muscle cells (RASMCs) were isolated and grown in phenol red free Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS). Confluent monolayers of RASMCs at passages four through eight were stimulated in serum-free media with IL-1β at doses encountered in human aortic aneurysms (2ng/mL).[12] After 48 hours of stimulation, culture media was collected and mRNA was extracted from the RASMCs. Following reverse transcription to cDNA, gene expression of MMP2 and TIMP2 (Tissue Inhibitor of MMP2; a major MMP2 inhibitor) was measured by real-time polymerase chain reaction. MMP2 protein levels in the conditioned media were measured by Western blotting, while MMP2 activity and TIMP2 activity were quantified by standard and reverse gelatin zymography. The remaining SMCs were lysed in 1% sodium dodecyl sulfate (SDS) and total cellular protein was determined by a bicinchoninic acid protein assay (BCA) (Pierce, Rockford, IL).
Experiment II
Male and female RASMCs (N= 6 repeated in triplicate) were incubated for 48 hours in phenol red free DMEM containing 17-β-estradiol and IL-1β. The five increasing concentration levels of 17-β-estradiol (1×10−10 to 1×10−6 molar) included both rat and human female physiologic levels of 17-β-estradiol. MMP2 activity in the conditioned media was determined by standard zymography.
Experiment III
Male Sprague-Dawley rats (190–210g) were again obtained from Charles River Laboratories (Wilmington, MA). Under general inhalational anesthesia (isofluorane 1.5%), 21-day sustained-release 17-β-estradiol pellets (0.1mg/pellet, Innovative Research of America, Sarasota, FL) were implanted in the interscapular subcutaneous plane. This particular estradiol dose results in serum estradiol levels at 2 to 3 weeks of 44.7 ± 6.1 pg/mL compared to 15.0 ± 2.2 pg/mL in control rats (P<0.05)[13]. Three weeks after pellet implantation, the infrarenal aorta was explanted under general anesthesia. The aorta was briefly rinsed free of blood and debris with cold 1× PBS containing antibiotics, cut into 1mm rings, and incubated for 48 hours in 1mL of serum-free DMEM containing antibiotics and IL-1β at 37° C. Following the incubation period, the conditioned media was collected for MMP2 activity analysis by standard gelatin zymography as follows. Tissue pieces were incubated for 24 hours at 37°C in 500µL of 1% SDS to extract protein which was subsequently assayed by BCA as follows.
Substrate gel zymography and reverse zymography
Zymography supplies were purchased from Novex (San Diego, CA). MMP distribution after treatment of RASMC with IL-1β and increasing concentrations of 17-β-estradiol was determined. Gelatin substrate zymograms were prepared using pre-cast 10% SDS-polyacrylamide gels containing 1 mg/mL of gelatin. Equal volumes of experimental media samples were diluted into 2× tris-glycine SDS sample buffer and electrophoretically separated under non-reducing conditions. Proteins were renatured in 2.7% Triton ×-100 and the gels were developed overnight at 37°C in 50 mM tris-HCl containing 5 mM CaCl2 and 2% Brij 35. Following overnight staining with Coomassie Blue R-250 and de-staining for four hours with 10% acetic acid and 40% methanol in water, gelatinase activity was evident by clear bands against a dark blue background.
Reverse zymography was performed with RASMC-conditioned media samples. Gelatin substrate reverse zymograms were prepared using a 15% acrylamide resolving gel containing 1mg/mL porcine gelatin and conditioned serum-free medium from separate RASMC cultures as a source of pro-gelatinase A. A standard 5% polyacrylamide stacking gel was used. Experimental samples containing equal volumes were diluted into 2× tris-glycine SDS sample buffer and electrophoretically separated under non-reducing conditions. Proteins were renatured in two changes of 2.7% Triton ×-100 for 60 minutes each. The gels were developed for 24 hours at 37°C in 50 mM Tris-HCl, 5 mM CaCl2 and 0.2% Brij 35. Following overnight staining with Coomassie Blue R-250 and de-staining for four hours with 10% acetic acid and 40% methanol in water, gelatinase inhibitory activity was evident as a blue band against a clear background.
The TIMP2 band was determined by comparison with authentic TIMP2 (29 kD) [22kD] obtained from Calbiochem (San Diego, CA). Semi-quantitative measurements of TIMP2 activity were performed by densitometry and corrected to total cellular protein.
Western blot analysis
Electrophoresis and Western blotting supplies were obtained from BioRad (Hercules, CA). Equal volumes of media from IL-1β-stimulated RASMC cultures were electrophoretically separated on a 7.5% acrylamide gel and blotted onto nitrocellulose membranes. Non-specific binding was blocked by incubating the membrane overnight in 20 mM tris-HCl (pH 7.5) containing 0.5 M NaCl, 0.1% Tween 20 and 5% nonfat milk. The primary antibody was monoclonal mouse anti-rat antibody to MMP2 (Calbiochem, San Diego, CA). Peroxidase-coupled goat anti-mouse antibody was used as a secondary antibody (Calbiochem). Immunoreactive bands were visualized using an ECL chemiluminescence detection kit from Amersham (Piscataway, NJ), and the amount of protein (corrected to total cellular protein) was measured by densitometry.
Semi-quantitative reverse transcriptase-polymerase chain reaction
Expression of MMP2 and TIMP2 messenger RNA (mRNA) was determined using semi-quantitative real-time polymerase chain reaction (RT-PCR). RASMCs treated with IL-1β were lysed and total cellular RNA was extracted using TRIzol reagent from Life Technologies (Rockville, MD), and mRNA was purified. Messenger RNA samples were reverse transcribed for 60 minutes at 42°C using an oligo-(dT) primer and Moloney’s murine leukemia virus (M-MLV) reverse transcriptase (Life Technologies). RT products were used as the substrate for PCR amplification of MMP2, TIMP2, and β-actin cDNAs with 5 U/mL Taq DNA polymerase from Promega (Madison, WI). Cycling was performed at 94°C for 2 minutes, followed by 32 cycles of 94°C for 1 minute, annealing at 57°C for 1 minute, and extension at 72°C for 1 minute, and a final incubation at 72°C for 5 minutes. Amplification was carried out in a GeneAmp 2400 PCR system from Perkin-Elmer (Foster City, CA). Primers were designed by Primer Premier Software (Premier Biosoft International, Palo Alto, CA), and were obtained from Sigma Genosys (Houston, TX). The sequences were as follows:
MMP2 sense 5'- CAT CGC TGC ACC ATC GCC CAT CAT C -3';
MMP2 antisense 5'- CCC AGG GTC CAC AGC TCA TCA TCA TCA AAG -3';
TIMP2 sense 5’- TGC CCT ATG ATC CCA TGC TA -3’;
TIMP2 antisense 5’- TCT GTG ACC CAG TCC ATC CA -3’;
β-actin sense 5'-ATG GGT CAG AAG GAT TCC TAT GTG-3';
β-actin antisense 5'-CTT CAT GAG GTA GTC AGT CAG GTC-3'.
The MMP2 primers were purchased from Sigma Genosys; TIMP2 and β-actin primers were purchased from Integrated DNA Technologies, Inc. (Coralville, IA).
Densitometry
All gel images were acquired using a FOTO/Analyst CCD CAMERA from Fotodyne (Hartland, WI). Band strength was quantified using GEL-Pro Analyzer software version 3.1 from Media Cybernetics (Silver Spring, MD). In cell culture experiments, only pro-MMP2 bands were observed.
Data Analysis
All experiments were performed in triplicate or quadruplicate. An unpaired, two-tailed Student’s t-test was used to determine differences in MMP2 and TIMP-2, with P< .05 considered significant. Statistical calculations were carried out using GraphPad Prism version 3.0a for Macintosh (GraphPad Software, San Diego, CA).
RESULTS
Experiment I – Gender related differences
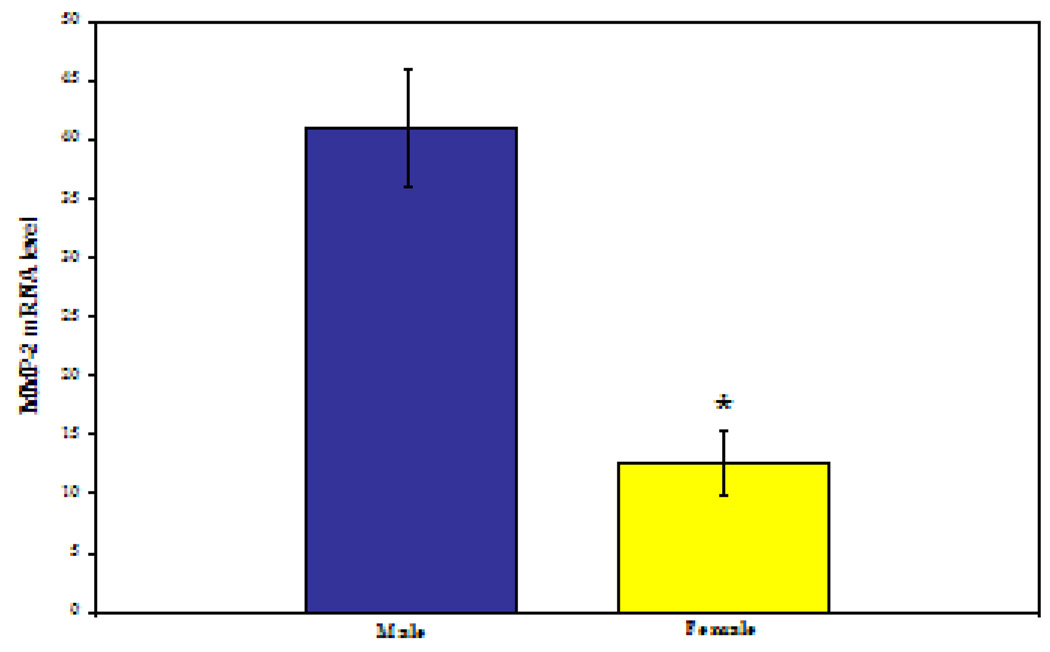
Intrinsic gender-related differences were observed between male and female RASMCs with regard to MMP2 gene expression, protein production, and gelatinolytic activity. MMP2 gene expression relative to β-actin was 3-fold higher in male compared to female IL-1β stimulated RASMCs (40.93 ± 4.9 vs. 12.54±2.7; P<0.0001, see Figure 1).
Figure 1.
MMP2 mRNA level by gender. As measured by real-time polymerase chain reaction, relative to β-actin mRNA level. * P<0.0001. (N=6 per group repeated in triplicate)
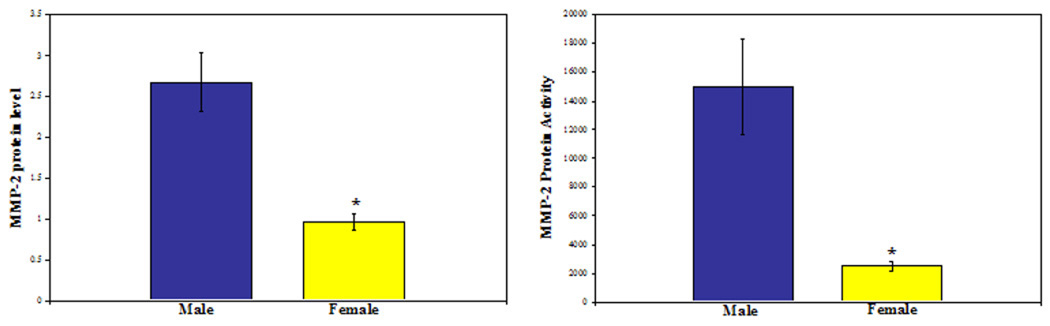
TIMP2 gene expression was greater in female than in male RASMCs (12.52 ± 3.6 vs. 5.4 ± 0.81; P<0.05), and the corresponding MMP2:TIMP2 gene expression ratio was greater in male vs. female cells (7.57 vs. 1.00, respectively). By Western blotting, MMP2 protein levels (O.D./mg total protein) were 3-fold higher (2.68±0.36 vs. 0.96±0.10) in male vs. female RASMCs (P=0.003), see Figure 2. Gelatinolytic activity by standard gelatin zymography (O.D./mg total protein) was more than 6-fold higher (15,010±3318vs. 2,472±363) in male vs. female cells (P=0.002), see Figure 2.
Figure 2.
MMP2 protein level and activity by Western blotting (*P=0.0003) and gelatin zymography (* P=0.002), respectively. (N=6 per group repated in triplicate)
Experiment II --Treatment of RASMCs with exogenous Estradiol
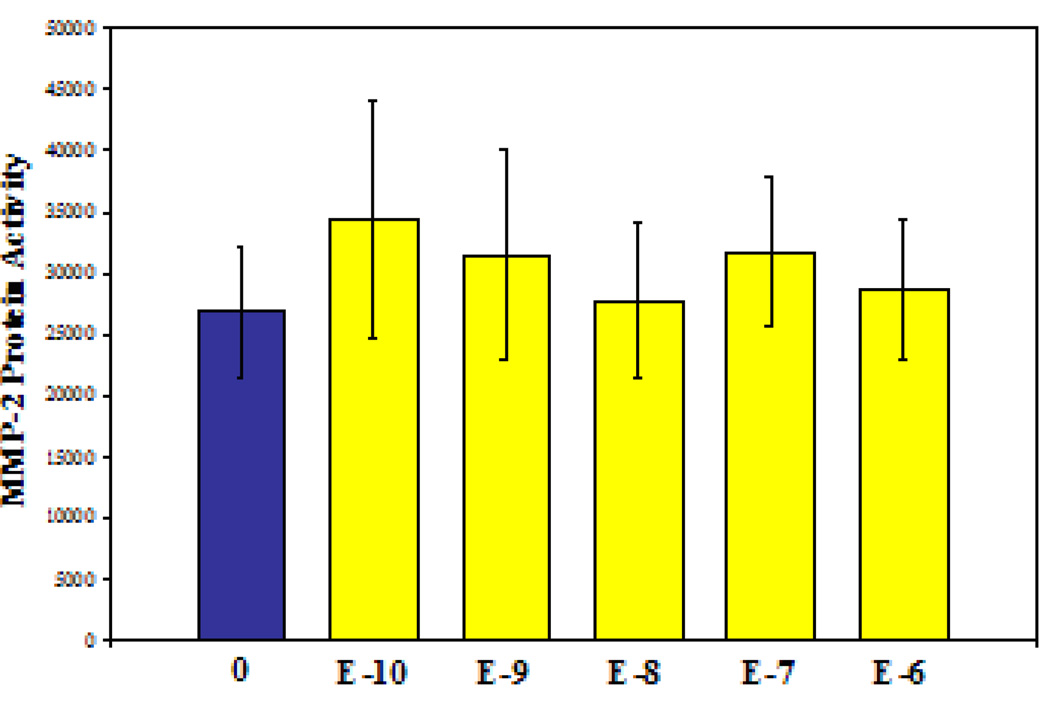
After observing apparent intrinsic differences in MMP2 and TIMP2 across gender, the second set of experiments sought to determine if MMP2 levels could be pharmacologically manipulated by co-culture with 17-β-estradiol. However, despite culture conditions that reflected physiologic female levels of estradiol for both rats [14] and humans [15], significant differences in MMP2 activity (by gelatin zymography) were not observed in male RASMCs. (Figure 3) Similar results were documented in female RASMCs; namely, that exogenous supplementation of 17-β-estradiol did not alter secretion of pro-MMP2 (P=0.9493).
Figure 3.
MMP2 activity was not significantly altered when male RASMCs were treated with 17-β-estradiol. The first column is no estradiol; the remaining columns are increasing 17-β-estradiol concentrations which include rodent and human female physologic levels. . (N=6 per group repated in triplicate)
Experiment III--in vivo estradiol therapy
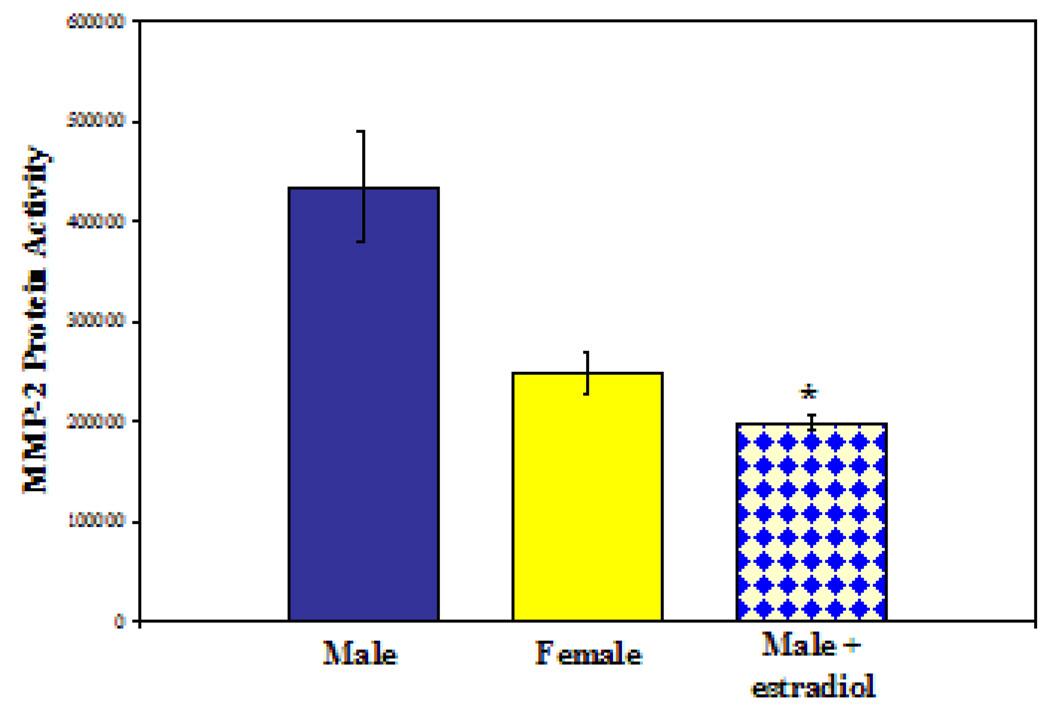
Given the inability of in vitro hormone alterations to alter MMP2 activity, we wished to determine if 17-β-estradiol may alter MMP2 activity in the more complex in vivo system. After pre-treatment with extended-release 17-β-estradiol pellets, male rats had a 2-fold decrease in MMP2 activity (O.D./mg total protein) in the media of whole-aortic explants (2.0×105 estradiol-treated vs. 4.35×105 control, P=0.002). By further comparison to controls, MMP2 activity in estradiol-treated males was found to be similar to female MMP2 activity (2.0 ×105 vs. 2.5×105; P=0.06, see Figure 4).
Figure 4.
MMP2 activity in explanted aortas from male, female, and male+estradiol rats. *P=0.002; male vs. male+estradiol.
Discussion
MMP2 (also known as Gelatinase-A and 72kDa Gelatinase) has been repeatedly associated with AAA pathogenesis. MMP2 is constitutively expressed by vascular smooth muscle cells[1], and it is increased in human[2–4] and murine AAAs.[5] Additionally, MMP2 knockout mice are highly resistant to experimental aneurysm formation.[6] Despite these repeated and consistent associations, MMP2 is certainly only a part of AAA pathogenesis.[16] Indeed, it is no longer believed that a single matrix metalloproteinase—be it MMP2, 9, 12, 14, or others—is the sole agent responsible for AAA formation.[16, 17] As suggested by Longo et al,[6] it is possible that multiple MMPs work in concert when remodeling (appropriately or pathologically) the aortic wall. Further, it is no longer believed that MMPs are necessarily the proximate damaging agent to the aortic wall. More likely, MMPs are involved in a complicated system of matrix recognition, immune modulation[18] and vascular remodeling. A recent study from our laboratories demonstrated that male rats which underwent neutrophil depletion were protected from AAA formation despite no difference in (single-gender) MMP2 levels.[19]
The present study is the first to characterize fundamental gender differences in MMP2 expression, protein production and protein activity in rat aortic smooth muscle cells. It has been long recognized that men suffer an increased incidence of aneurysm formation[11, 20], but the reasons for this gender-related disparity are not known. Paralleling differences in AAA susceptibility, this investigation demonstrates that male aortic mesenchymal cells produce significantly greater amounts of MMP2 than do their female counterparts. In addition, the ratio of MMPs and their inhibitors, the TIMPs have been implicated in aortic disease. Manabe et al.[21] reported that the TIMP2:MMP2 ratio is decreased in the acute phase of aortic dissections, which is consistent with our observations of increased MMP2:TIMP2 ratio in the aneurysm-susceptible male gender.
This investigation provides another molecular example to support the observation that certain subsets of the general population are more susceptible to diseases than others. At baseline, MMP2 levels in female RASMCs is less than in male counterparts, and these differences are alterable by in vivo hormone administration. This is a highly localized phenomenon (in abdominal aortic smooth muscle cells); it does not necessarily stand in contradiction to recent reports of equal serum concentrations of MMP2 across human gender.[22] Regardless, it is doubtful that MMP2 will be used in screening for AAA, as serum levels are an insensitive marker for AAA presence or expansion.[23, 24]
It was observed in this investigation that estradiol influences MMP2 levels in vivo, and that baseline gender differences may be ameliorated by hormone modulation. This relationship has been observed in other areas of cardiovascular investigation. Chancey et al[25] report increased susceptibility to cardiac dysfunction in estrogen-deficient (ovariectomized) female animals which corresponded to increased MMP2 levels. In corresponding randomized clinical trials, however, transdermal estrogen supplementation failed to alter serum MMP2 levels.[26]
Conclusions
MMP2 gene expression, protein levels, and gelatinolytic activity were higher in male compared to female RASMCs. Exogenous 17-β-estradiol did not alter MMP2 activity in vitro in male or female SMCs, but in vivo 17-β-estradiol exposure greatly decreased male aortic MMP2 production. Gender differences in MMP2 are speculated to be associated with phenotypic differences in human and rat AAA formation. Future investigations in AAA formation will certainly reveal many correlations between gender, molecular-level pathogenesis, and susceptibility to AAA.
Acknowledgments
Supported by: NIH T32 (HL076123) (DTW), NIH KO8 (HL67885-02) (GRU), VonLeibig Award-Lifeline Foundation (GRU), 5R01(HL081629-02) (GRU).
Footnotes
Portions of this work were presented at the 2005 Midwest Vascular Surgical Society in Chicago, IL
Bibliography
- 1.Crowther M, et al. Increased matrix metalloproteinase 2 expression in vascular smooth muscle cells cultured from abdominal aortic aneurysms. J Vasc Surg. 2000;32(3):575–583. doi: 10.1067/mva.2000.108010. [DOI] [PubMed] [Google Scholar]
- 2.Goodall S, et al. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation. 2001;104(3):304–309. doi: 10.1161/01.cir.104.3.304. [DOI] [PubMed] [Google Scholar]
- 3.Crowther M, et al. Localization of matrix metalloproteinase 2 within the aneurysmal and normal aortic wall. Br J Surg. 2000;87(10):1391–1400. doi: 10.1046/j.1365-2168.2000.01554.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis V, et al. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1998;18(10):1625–1633. doi: 10.1161/01.atv.18.10.1625. [DOI] [PubMed] [Google Scholar]
- 5.Sinha I, et al. Early MT-1 MMP expression following elastase exposure is associated with increased cleaved MMP2 activity in experimental rodent aortic aneurysms. Surgery. 2004;136(2):176–182. doi: 10.1016/j.surg.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Longo GM, et al. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110(5):625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treharne GD, et al. Marimastat inhibits elastin degradation and matrix metalloproteinase 2 activity in a model of aneurysm disease. Br J Surg. 1999;86(8):1053–1058. doi: 10.1046/j.1365-2168.1999.01196.x. [DOI] [PubMed] [Google Scholar]
- 8.Patel MI, et al. Increased synthesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms. J Vasc Surg. 1996;24(1):82–92. doi: 10.1016/s0741-5214(96)70148-9. [DOI] [PubMed] [Google Scholar]
- 9.Katz DJ, Stanley JC, Zelenock GB. Abdominal aortic aneurysms. Semin Vasc Surg. 1995;8(4):289–298. [PubMed] [Google Scholar]
- 10.Katz DJ, Stanley JC, Zelenock GB. Gender differences in abdominal aortic aneurysm prevalence, treatment, and outcome. J Vasc Surg. 1997;25(3):561–568. doi: 10.1016/s0741-5214(97)70268-4. [DOI] [PubMed] [Google Scholar]
- 11.Ailawadi G, et al. Gender Differences in Experimental Aortic Aneurysm Formation. Arterioscler Thromb Vasc Biol. 2004 doi: 10.1161/01.ATV.0000143386.26399.84. [DOI] [PubMed] [Google Scholar]
- 12.Davis VA, et al. Cytokine pattern in aneurysmal and occlusive disease of the aorta. J Surg Res. 2001;101(2):152–156. doi: 10.1006/jsre.2001.6281. [DOI] [PubMed] [Google Scholar]
- 13.Brooks-Asplund EM, et al. Estrogen has opposing effects on vascular reactivity in obese, insulin-resistant male Zucker rats. J Appl Physiol. 2002;92(5):2035–2044. doi: 10.1152/japplphysiol.00559.2001. [DOI] [PubMed] [Google Scholar]
- 14.Tou JC, et al. The effect of purified compared with nonpurified diet on bone changes induced by hindlimb suspension of female rats. Exp Biol Med (Maywood) 2005;230(1):31–39. doi: 10.1177/153537020523000104. [DOI] [PubMed] [Google Scholar]
- 15.Holmberg L, et al. Pre-operative oestradiol levels - relation to survival in breast cancer. Eur J Surg Oncol. 2001;27(2):152–156. doi: 10.1053/ejso.2000.1084. [DOI] [PubMed] [Google Scholar]
- 16.Ailawadi G, Eliason JL, Upchurch GR., Jr Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg. 2003;38(3):584–588. doi: 10.1016/s0741-5214(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 17.Parks WC. A confederacy of proteinases. J Clin Invest. 2002;110(5):613–614. doi: 10.1172/JCI16550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannawa KK, et al. L-selectin-mediated neutrophil recruitment in experimental rodent aneurysm formation. Circulation. 2005;112(2):241–247. doi: 10.1161/CIRCULATIONAHA.105.535625. [DOI] [PubMed] [Google Scholar]
- 19.Eliason JL, et al. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005;112(2):232–240. doi: 10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- 20.National Hospital Discharge Survey Data. National Center for Health Statistics [Google Scholar]
- 21.Manabe T, et al. Decreased tissue inhibitor of metalloproteinase-2/matrix metalloproteinase ratio in the acute phase of aortic dissection. Surg Today. 2004;34(3):220–225. doi: 10.1007/s00595-003-2683-3. [DOI] [PubMed] [Google Scholar]
- 22.Tayebjee MH, et al. Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and -9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and -2. Thromb Res. 2005;115(3):205–210. doi: 10.1016/j.thromres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson P, et al. Genotype-phenotype relationships in an investigation of the role of proteases in abdominal aortic aneurysm expansion. Br J Surg. 2005 doi: 10.1002/bjs.5126. [DOI] [PubMed] [Google Scholar]
- 24.Eugster T, et al. Aminoterminal propeptide of type III procollagen and matrix metalloproteinases-2 and -9 failed to serve as serum markers for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2005;29(4):378–382. doi: 10.1016/j.ejvs.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Chancey AL, et al. Modulation of cardiac mast cell-mediated extracellular matrix degradation by estrogen. Am J Physiol Heart Circ Physiol. 2005;289(1):H316–H321. doi: 10.1152/ajpheart.00765.2004. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson JC, et al. Randomized trial of effect of transdermal continuous combined hormone replacement therapy on cardiovascular risk markers. Br J Haematol. 2004;124(6):802–808. doi: 10.1111/j.1365-2141.2004.04846.x. [DOI] [PubMed] [Google Scholar]