Figure 1.
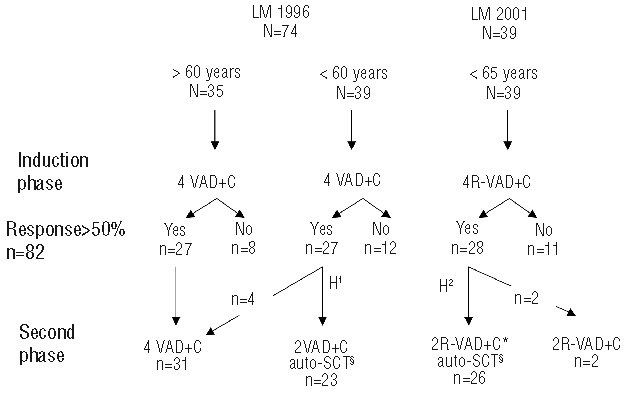
Study design of the LM 1996 and LM 2001 trials and number of responders at the intermediate and final step of evaluation. N= number of patients recruited in each arm. VAD+C: vincristine 0.4 mg/D, D1 to D4; doxorubicin 9 mg/m2/D, D1 to D4; dexamethasone 40 mg/D, D1 to D4; chlorambucil 12 mg/D, D20 to D29 ; 2 cycle delay 35 days. RVAD+C: rituximab 375 mg/m2, D1 of each VAD cycle. H1 and H2: stem cell harvest; H1, steady state manner; H2, cyclophosphamide mobilization 4 g/m2 and in vitro purge with rituximab 375 mg/m2. 2(R)VAD+C*, one RVAD+C followed by one VAD+C. §Melphalan 140 mg/m2 and TBI 8 gy/4 fractions.
