Figure 1.
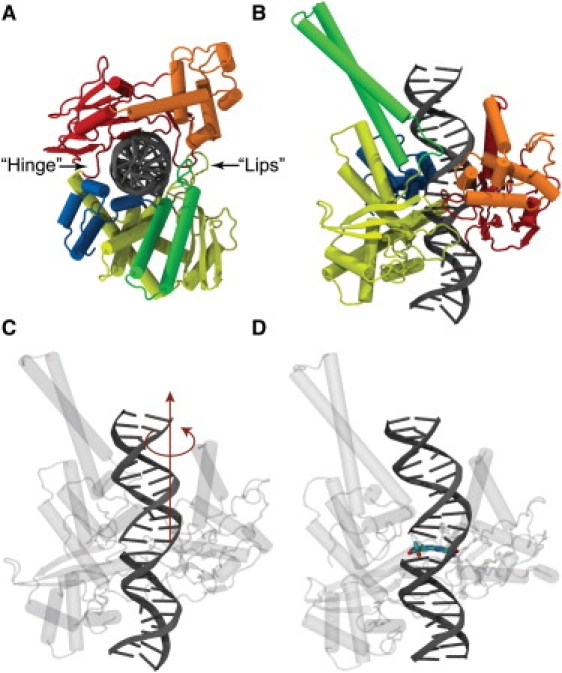
Human topoisomerase I in complex with DNA. Protein is composed of four domains: the N-terminal (not resolved in crystal structures); the core domain (which is divided into three subdomains shown in red, orange, and yellow); the linker domain (shown in green); and the C-terminal domain (in blue). (A) View down the DNA axis. Protein resembles a Pac-Man with an upper cap and a lower base: subdomain I and II of the core form the upper cap, and subdomain III, together with the C-terminal domain, form the lower base of the Pac-Man. The Pac-Man-like enzyme opens its lips to remove positive supercoils and stretches its hinge when removing negative ones (see text). (B) View of the complex perpendicular to the DNA axis. A 22-basepair DNA segment is shown in gray. (C) The swiveling axis about which the DNA duplex downstream of the cut is rotated; the downstream DNA is actually running upward in this snapshot. (D) Same snapshot as in panel c, with the drug topotecan shown in its crystal-structure position, intercalating between the two DNA basepair stack flanking the nick, which displaces the rotating DNA part one flight up and imposes steric constraints during DNA rotation.
