Figure 1.
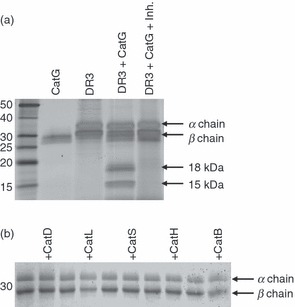
Specific proteolysis of human leukocyte antigen (HLA)-DR mediated by cathepsin G (CatG) in vitro. (a) HLA-DR3 (DR3), affinity purified from DR3 transfectants of the HLA-DM-negative B-cell line 5.2.4, was incubated for 2 hr at 37° and pH 7 with CatG in the presence or absence of a CatG inhibitor. The degradation products were visualized by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining. Data are representative of five independent experiments. (b) Lack of DR3 proteolysis by other cathepsins. DR3 molecules purified from the HLA-DM-deficient B-cell line 9.5.3 were incubated with the indicated cathepsins at pH 5 for 2 hr before SDS-PAGE and Coomassie Blue staining. Data are representative of two independent experiments. Inh., CatG inhibitor.
