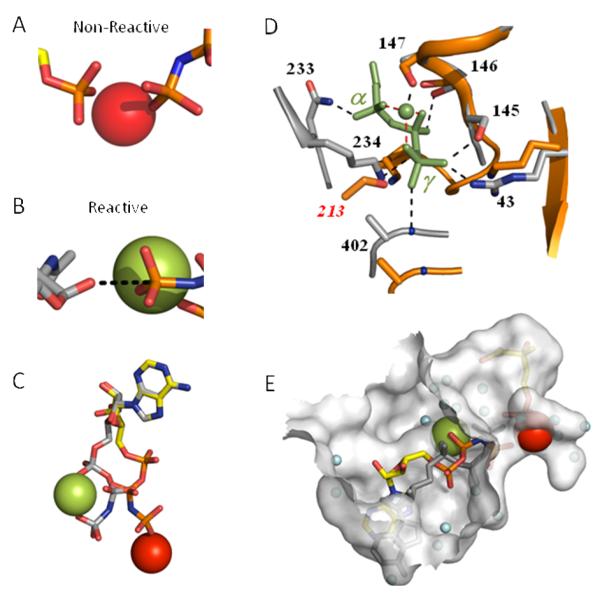
Figure 5. Type I and II ligand conformations in GHMP kinase ternary structures.
(A) The Type I conformation. The positioning of the γ- and Pmev-phosphoryl groups in the PMK ternary complex (shown on the left and right-hand sides of the panel, respectively) suggests that they are non-reactive. Similiar conformations are found in three of the seven GHMP-kinase ternary complex structures. (B) The Type II conformation. Positioning of the reactive moieties in the ternary complex of Gal-NAc kinase — a GHMP kinase. The Gal-NAc nucleophillic oxygen and the γ-phosphoryl group of AMPPNP appear to be positioned well to accomplish in-line displacement. This orientation is also found in three of the seven GHMP kinase ternary complex structures. (C) Alignment of the Type I and II M2+·nucleotides. The adenine bases of the Type I and II nucleotides were aligned. The PMK nucleotide color coding: carbon (yellow), phosphorus (orange), divalent cation (red); the Gal-NAc kinase nucleotide color coding: carbon and phosphorus (grey), divalent cation (green). (D) Comparison of the Type I and II active-sites. Resides (grey) within 3 Å of the PPPi·Mg2+ moiety (green) of Glc-NAc kinase complex (Type II) are linked to the moiety by blank dotted lines. Analogous residues in the PMK complex (Type I) are shown in orange. Dashed red lines connect the divalent cation to the α, β and γ-phosphoryl groups to which it is coordinated. (E) Positioning the Type II nucleotide·M2+ in the Type I active site. The Type I and II active-sites were superposed using Cα-alignments of conserved regions of PMK and Gal-NAc kinase. The GalNac nucleotide (Type II) is shown in grey and its associated cation (Mn2+) is red. The transition from Type I to II requires the cation to migrate across the active-site cavity. Blue spheres represent ordered water. Figures were produed using PyMol (54) and the surface, shown in E, was created with Hollow (56).