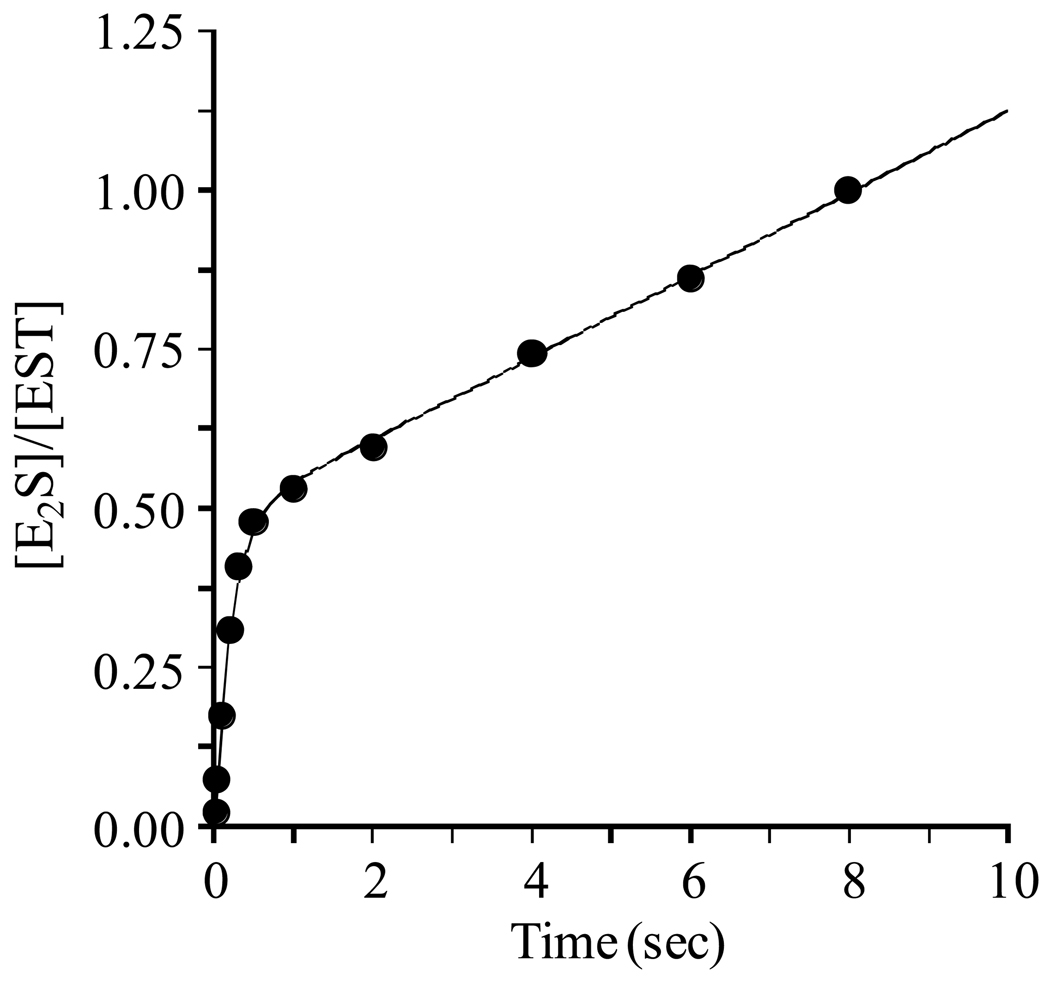
Figure 1. A burst of E2S formation.
E2S synthesis was initiated by rapidly mixing a solution containing EST (1.0 µM, dimer), [3H]E2 (3.6 µM, 720 × Km, SA = 90 Ci/mmol), glycerol (10% v/v), MgCl2 (7.0 mM), DTT (1.0 mM), and 50 mM KPO4 (pH 6.3) with a solution of equal volume that was identical except that it lacked EST and E2, and contained PAPS (18.0 µM, 305 × Km). Reactions were quenched by rapidly mixing the reacting solutions with an equal volume of HCl (0.66 M). [3H]E2 was extracted from the quenched mixture using CCl4 and [3H]E2S, which remained in the aqueous phase, was counted (see Materials and Methods). All solutions were equilibrated at 25 (± 2) °C prior to mixing. Reactions were performed in triplicate and averaged. The smooth curve represents the best-fit of the averaged data to the equation: A0 (1–e−λt) + kcatt.