Figure 5.
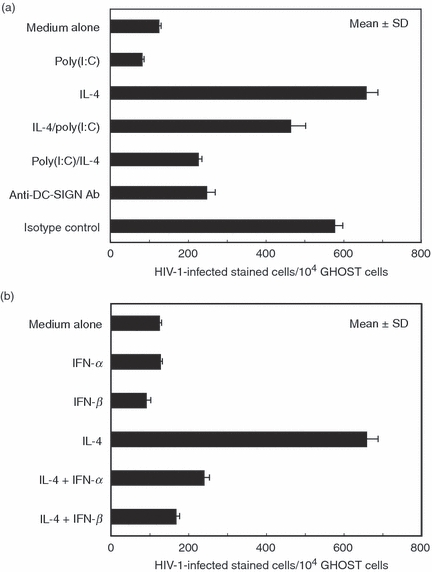
Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM3) grabbing nonintegrin (DC-SIGN)-mediated transmission of human immunodeficiency virus type 1 (HIV-1) via breast milk macrophages (BrMMø) was inhibited by treatment not only with poly(I:C) (a) but also with interferons (IFNs) (b). (a) To examine the effect of poly(I:C) treatment on DC-SIGN-mediated HIV-1 transmission, BrMMø incubated for 24 hr with 50 μg/ml poly(I:C) were further incubated with either culture medium or interleukin (IL)-4 for an additional 4 days after extensive washing to remove free poly(I:C). Also, BrMMø co-cultured with IL-4 for 4 days were further incubated with 50 μg/ml poly(I:C) for the final 24 hr. These poly(I:C)-treated BrMMø were infected with a 0·2 multiplicity of infection (MOI) dose of the R5-type macrophage-tropic HIV-1 isolate NL(AD8) for 2 hr at 37°. After extensive washing to remove free NL(AD8) virions, 1 × 104Ghost X4/R5 cells were added to NL(AD8)-pulsed BrMMø and incubated for an additional 16 hr. After incubation, the HIV-1-loaded BrMMø were gently removed with warmed D-10, and NL(AD8)-infected Ghost X4/R5 cells expressing green fluorescent protein (GFP) were analysed by flow cytometry to estimate transmissibility. Also, to examine the possibility of a DC-SIGN-independent pathway for HIV-1 transmission by BrMMø, IL-4-stimulated BrMMø pretreated with 20 μg of anti-DC-SIGN monoclonal antibody (mAb) for 30 min on ice were infected with NL(AD8) for 2 hr at 37° and added to Ghost X4/R5 cells. (b) Similarly, to investigate the effect of IFNs on DC-SIGN-mediated HIV-1 transmission, BrMMø co-cultured with IFNs and IL-4 for 5 days were infected with a 0·2 MOI dose of NL(AD8) for 2 hr at 37°, washed to remove free virions, and incubated with 1 × 104Ghost X4/R5 cells for an additional 16 hr before analysis by flow cytometry. Data are representative of at least four experiments and the results are expressed as the mean ± standard deviation.
