Figure 2.
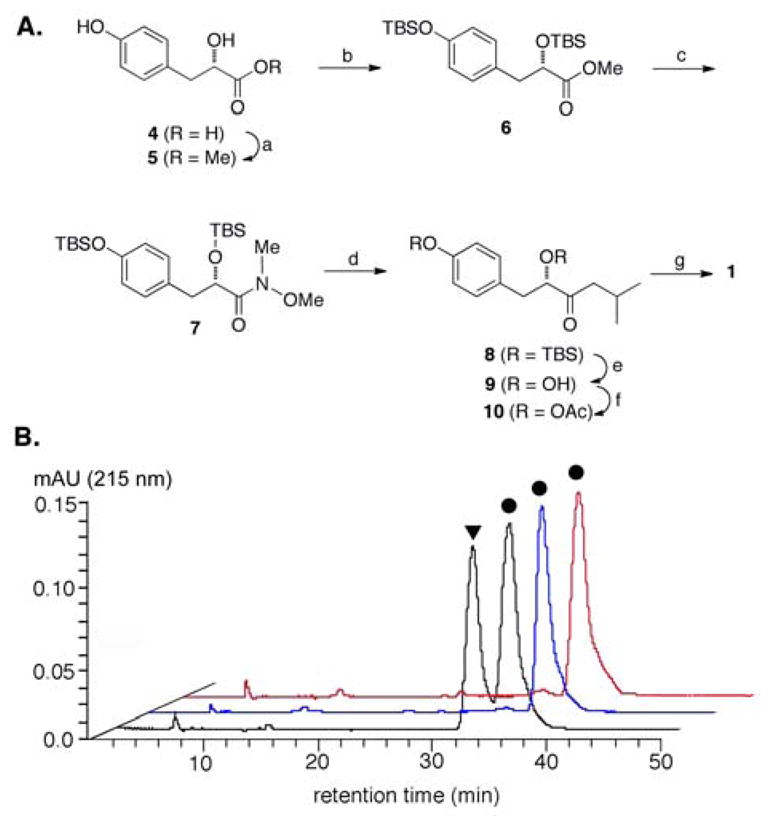
Synthesis and chiral HPLC analysis of natural vs. synthetic 1: (A) reagents and conditions for total synthesis of 1 from the optically pure starting material 4 [(a) MeOH, HCl, rt. quantative (b) TBSCl, imidazole., DMF, rt. 90%; (c) HN(OMe)CH3-HCl, (CH3)2CHMgCl, THF, 0 °C, 81%; (d) (CH3)2CHCH2MgCl, THF, 0°C-rt, 67%; (e) TBAF, THF, rt. 86%; (f) Ac2O, pyridine, rt. 72%; (g) pyrrolidine, 1 min., rt. 66%] and (B) chiral HPLC analysis of racemic 1, 1 from A rutilus (blue), synthetic 1 (red). ●, 1, and ▼ the R-enantiomer of 1.
