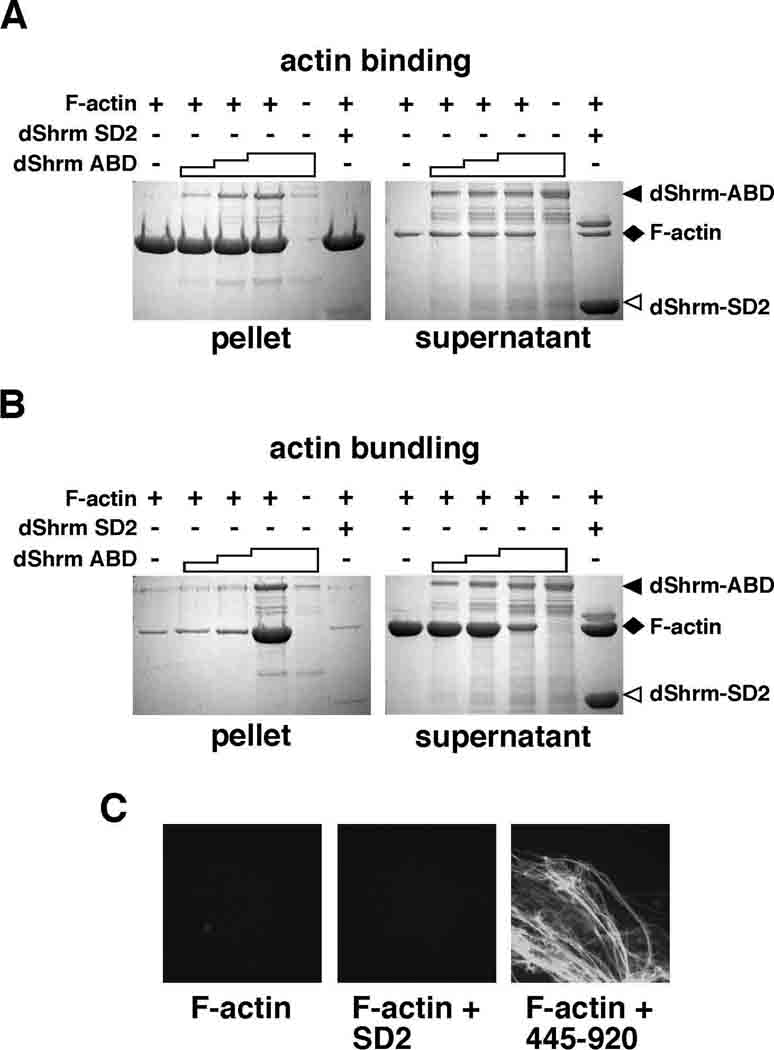
Figure 4. dShrmA directly binds to F-actin.
(A) For actin binding assays, increasing amounts of dShrmA proteins consisting of amino acids 445–920, containing the putative Actin Binding Domain (dShrm-ABD) was mixed with purified F-actin and then F-actin was centrifuged at high speed to pellet all actin filaments and any associated proteins. Supernatant and pellet fractions were resolved by SDS-PAGE and detected by Coomassie staining. Staircase denotes increasing amounts of dShrm-ABD added while + and − signs denote the presence or absence of the indicated protein in the reaction. dShrm-SD2, dShrm-ABD alone, and F-actin alone are shown as controls.
(B and C) dShrmA can induce the formation of actin aggregates. Actin binding experiments were repeated as in A, but F-actin was centrifuged at low speed to pellet only actin aggregates. In C, samples were removed prior to centrifugation, incubated with FITC-phalloidin, and visualized by confocal microscopy.