Table 2.
Synthesis of tetrahydroquinoline analogs
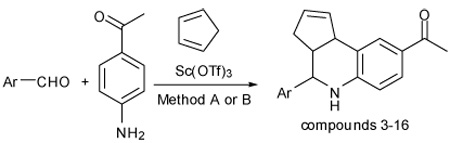 | ||||||
|---|---|---|---|---|---|---|
| Entry | Aldehyde | Methoda,b | Time(h) | Product | Yield(% )c | endo/exod |
| 1 |  |
A | 2.0 | 3 | 88 | 93:7 |
| 2 | 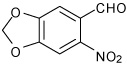 |
A | 3.0 | 4 | 95 | 95:5 |
| 3 | 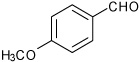 |
A | 5.0 | 5 | 75 | 91:9 |
| B | 2.5 | 5 | 90 | 92:8 | ||
| 4 | 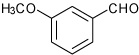 |
A | 2.0 | 6 | 95 | 92:8 |
| 5 | 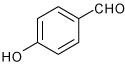 |
A | 5.0 | 7 | 70 | 95:5 |
| B | 15.0 | 7 | 80 | 96:4 | ||
| 6 | 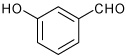 |
A | 1.5 | 8 | 95 | 96:4 |
| 7 | 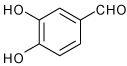 |
A | 5.0 | 9 | 70 | 92:8 |
| B | 15.0 | 9 | 72 | 92:8 | ||
| 8 |  |
A | 2.0 | 10 | 77 | 93:7 |
| 9 | 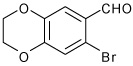 |
A | 3.0 | 11 | 84 | 92:8 |
| 10 | 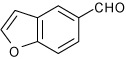 |
A | 3.0 | 12 | 92 | 92:8 |
| 11 | 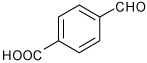 |
A | 2.5 | 13 | 92 | 92:8 |
| 12 |  |
A | 1.0 | 14 | 98 | 89:11 |
| 13 | 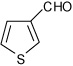 |
A | 1.5 | 15 | 94 | 80:20 |
| 14 | 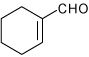 |
A | 1.0 | 16 | 90 | 63:37 |
A: Multicomponent procedure.
B: Stepwise imineformation, cyclization.
Isolated yields.
Endo/exo ratio determined by 1H NMR and HPLC.
