Abstract
Vaccination has become an important therapeutic approach to the treatment of Alzheimer’s disease (AD), however, immunization with Aβ amyloid can have unwanted, potentially lethal, side effects. Here we demonstrate an alternative peptide-mimotope vaccine strategy using the SDPM1 peptide. SDPM1 is a 20 amino acid peptide bounded by cysteines that binds tetramer forms of Aβ1–40- and Aβ1–42-amyloid and blocks subsequent Aβ amyloid aggregation. Immunization of mice with SDPM1 induced peptide mimotope antibodies with the same biological activity as the SDPM1 peptide. When done prior to the onset of amyloid plaque formation, SDPM1 vaccination of APPswePSEN1(A246E) transgenic mice reduced amyloid plaque burden and Aβ1–40 and Aβ1–42 levels in the brain, improved cognitive performance in Morris water maze tests, and resulted in no increased T cell responses to immunogenic or Aβ peptides or brain inflammation. When done after plaque burden was already significant, SDPM1 immunization still significantly reduced amyloid plaque burden and Aβ1–40/1–42 peptide levels in APPswePSEN1(A246E) brain without inducing encephalitogenic T cell responses or brain inflammation, but treatment at this stage did not improve cognitive function. These experiments demonstrate the efficacy of a novel vaccine approach for Alzheimer’s disease where immunization with an Aβ1–40/1–42 amyloid-specific binding and blocking peptide is used to inhibit the development of neuropathology and cognitive dysfunction.
Keywords: Alzheimer’s disease, aging, dementia, vaccine, peptide mimotope, memory, amyloid
Introduction
Alzheimer’s disease (AD) is the most prevalent form of dementia in the elderly(Bachman et al., 1992). The decline in cognitive abilities in AD is associated with pathologic changes in the brain, the most prevalent of which are neurofibrillary tangles and amyloid plaques(Selkoe, 2001). Amyloid plaques, which occur at far greater levels in AD brain than in normal individuals, are one of the most robust pathologic hallmarks of AD(Terry, 1996). Amyloid plaques are formed by the sequential cleavage of the amyloid precursor protein (APP) by β (BACE) and γ (Presenilin) secretases to create predominantly Aβ1–40 or Aβ1–42 peptides(Tabaton and Tamagno, 2007). Once made, these Aβ peptides aggregate to create Aβ amyloid and ultimately contribute to the formation of amyloid plaques in the brain. A number of animal models bearing mutations in APP or presenilin 1 (PSEN1), both genes that when mutated can cause early onset forms of AD(Selkoe, 2001), have been made that recapitulate amyloid plaque formation with associated synaptic and cognitive deficits(Games et al., 2006). These monogenic or bigenic transgenic models, however, do not recapitulate the formation of neurofibrillary tangles or neurodegeneration, two important aspects of AD pathology(Jaworski et al.). Aβ amyloid formation involves the formation of quaternary protein structures, beginning with the dimerization of the Aβ1–40 or Aβ1–42 peptide and subsequent oligomerization that ultimately creates very high molecular weight protein aggregates. Many studies have demonstrated that various low molecular weight Aβ oligomeric forms are toxic to neurons(Bard et al., 2003; Barghorn et al., 2005; Cleary et al., 2005; Deshpande et al., 2006; Gong et al., 2003; Lambert et al., 1998; Noguchi et al., 2009; Selkoe, 2001; Shankar et al., 2008). Aβ amyloid, being a pathologic protein structure, is also immunogenic; many AD patients have antibody titers against Aβ peptides or Aβ amyloid(Du et al., 2001; Geylis et al., 2005; Hyman et al., 2001; Nath et al., 2003; Weksler et al., 2002). Indeed, some studies suggest that AD patients have lower anti-Aβ antibody titers than healthy elderly individuals and that higher titers may therefore be protective against development of AD(Du et al., 2001; Weksler et al., 2002).
Consistent with this notion, one of the most promising approaches to treatment for AD has been to immunize patients with Aβ1–42 amyloid to stimulate immune clearance of amyloid plaques, an approach first described by Schenk and colleagues in 1999(Schenk et al., 1999). This approach has been repeatedly shown to stimulate clearance of amyloid plaque burden in the brain of AD mouse models as well as to increase performance on cognitive tasks and decrease synapse loss(Buttini et al., 2005; Morgan, 2006; Schenk et al., 2005). A clinical trial for AD (ClinicalTrials.gov:NCT00021723) using Aβ1–42 peptide containing QS21, a strong Th1-biased adjuvant (AN1792), however, was halted due to the unanticipated side effect of asceptic meningoencephalitis in 6% of vaccine recipients, which likely resulted from development of Aβ-specific Th1-type T lymphocytes(Orgogozo et al., 2003). Another issue in this trial was that only about 20% of vaccinated patients developed an increased antibody titer to Aβ1–42 despite the use of adjuvant, suggesting vaccine potency was either suboptimal or that the majority of patients had immune tolerance to Aβ amyloid(Gilman et al., 2005). Aβ amyloid vaccination did clear brain amyloid burden for at least some AD patients where vaccination had increased Aβ amyloid antibody titer(Nicoll et al., 2006). In addition, some AN1792-immunized subjects demonstrated relatively slower cognitive decline(Gilman et al., 2005; Hock et al., 2003). These two findings suggest that vaccine approaches to AD hold great promise.
The finding of brain inflammation as a side effect of AD vaccine therapy has led to a plethora of modified vaccine strategies designed to minimize T cell responses to the Aβ peptide while maintaining the therapeutic humoral response. These have focused on passive immunization, where therapeutic antibodies are directly administered(Bard et al., 2003; Hock et al., 2003; Lee et al., 2006; Pan et al., 2002; Tucker et al., 2008; Yamada et al., 2009), as well as modified active Aβ amyloid immunization strategies(Ghochikyan et al., 2006a; Ghochikyan et al., 2006b; Lemere, 2009; Lemere et al., 2007; Moretto et al., 2007; Schneeberger et al., 2009; Seabrook et al., 2007; Sigurdsson et al., 2004). Many of these latter approaches involve using shortened N-terminal fragments of the Aβ1–42 peptide that lack the predominant T cell epitopes (which occur in the more C-terminal region of Aβ1–42)(Kutzler et al., 2006; Monsonego et al., 2001; Monsonego et al., 2003). While such a modified active immunization approach is logical, vaccination using shortened Aβ peptides may also preclude the development of therapeutic humoral responses against certain quaternary Aβ amyloid forms, which may not be present in such immunogens. Another immunologic approach, the one we have taken here, would be to identify peptides that specifically bind to particular low molecular weight quaternary Aβ amyloid forms and use these to generate anti-ideotype-like immune responses (peptide mimotope antibodies(Riemer and Jensen-Jarolim, 2007)) that recognize the same Aβ amyloid structures as the immunogenic peptides. Here we show that immunization with SDPM1, an Aβ1–40/42 amyloid binding and blocking peptide, reduces neuropathology and improves cognitive function in mouse model of Alzheimer’s disease.
Methods
Animals
All experiments were done in accordance with the Guide for the Use and Care of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at Nationwide Children’s Hospital. APPswePSEN1(A246E) animals (B6C3-Tg(APP695)3Dbo Tg(PSEN1)5Dbo/J, stock #003378), originally made by Borchelt, Sisodia, and colleagues(Borchelt et al., 1997), and strain specific control littermates were obtained from the Jackson Laboratory (Bar Harbor ME) and bred and genotyped as before. These mice express human presenilin 1 (A246E) variant and a chimeric amyloid precursor protein (APPSwe), where mouse APP695 contains the human Aβ peptide domain including the Swedish (Swe) AD mutations K595N and M596L. Transgene expression is driven in neurons for both transgenes via the mouse prion protein (Prnp) promoter. Both transgenes were maintained as heterozygous alleles for all experiments. All animals were housed in a barrier facility and were housed individually prior to Morris Water Maze or Open Field test assessments.
Peptide binding experiments
Aβ1–40 and Aβ1–42 peptide (Sigma; St. Louis, MO) were stored frozen in DMSO was diluted into Tris buffered saline (TBS), pH 7.4, at 20µg/ml to form amyloid (as in (Kang et al., 2003)). Peptide was incubated in TBS for 7–10 days prior to use as amyloid in ELISA assays. For Aβ binding experiments, Aβ1–40 or Aβ1–42 amyloid were immobilized at 100ng/well in 50mM sodium bicarbonate buffer, pH 9.5, overnight at 4°C. Immobilization on nitrocellulose-coated ELISA plates was also done with similar results. Plates were blocked in Hank’s balanced salts (HBS) with 1mM CaCl2, 1mM MgCl2, 10mM HEPES pH 7.4, and 0.5% bovine serum albumin (HBS binding buffer). SDPM1 (AEC-DWGKGGRWRLWPGASGKTEA-CGP, synthesized and purified by AnaSpec, San Mateo, CA) or SDPM2 (DWGKGGRWRLWPGASGKTEA; AnaSpec, San Mateo, CA), conjugated with biotin at their N-terminus, were added at the indicated concentrations. For Aβ1–16, Aβ12–28, Aβ16–40, and Aβ16–42 blocking experiments (all from Sigma, St. Louis, MO), a 10-fold molar excess of peptide was mixed with SDPM1 in HBS binding buffer for one hour prior to the binding experiment. After washing in binding buffer, streptavidin conjugated with horseradish peroxidase (HRP) was added at 1:1000, followed by further washing and development of peroxidase activity, as before(Kang et al., 2003). Binding was followed as absorbance at 450nm on an ELISA plate reader (SpectraMax M2; Molecular Devices; Sunnyvale CA), at 5-minute time intervals for 30–60 minutes. Readings in the linear range (0.2–1.0) were used for analysis, as before. Binding of secondary reagents alone was negligible in most instances and never exceeded 10% of the primary signal.
Production of SDPM1-specific monoclonal antibodies
One hundred µg of SDPM1 peptide, containing an N-terminal biotin, was added to 25 µg of streptavidin (Sigma S0677) in 50µL of phospho-buffered saline (PBS, pH 7.4) overnight at 4°C to create the immunogenic antigen. Antigen was mixed with 50µL adjuvant and emulsified in a sonicator. One hundred µL of antigen/adjuvant was injected intraperiteoneally (using a 27-gauge needle) into 8 week-old Balb/c mice. For the first injection, TiterMax Gold adjuvant was used (Sigma T2684). Mice were boosted three times, once every two weeks, with the same amount of antigen (mixed with Freund’s incomplete adjuvant (Sigma F5506)). Two weeks after the final boost, SDPM1-specific serum titer was assessed and the two animals with the highest SDPM1 titer were used to make hybridoma cell lines.
Hybridomas were made by cell fusion of isolated splenocytes with a proprietary myeloma cell line by the Immunological Resource Center at the University of Illinois at Urbana-Champaign (by contract). Fused cells were subsequently selected using HAT (Hypoxanthine-aminopterin-thymidine) media supplement. Five hundred and seventy-six clones were screened for SDPM1-specific titers by comparing antibody binding to SDPM1-biotin-streptavidin, SDPM2-biotin-streptavidin, and streptavidin alone. Five to ten percent of clones on each 96-well plate showed binding to streptavidin, but not to SDPM1 or SDPM2, while several clones typically showed binding to both SDPM1 and SDPM2. Only two clones were identified that had significantly increased binding to SDPM1 relative to SDPM2. Examples in each category were subcloned at the single cell level and rescreened for binding. Isotypes of all mouse monoclonal antibodies used was determined (Southern Biotech 5300-05; Birmingham AL).
Amyloid formation and trapping assays
Thioflavin T (Fluka 88630) absorbance assays were done as previously described(Naiki et al., 1997). Equimolar amounts of Aβ1–40 or Aβ1–42 (50µM) were mixed with SDPM1 or SDPM2 (50µM) or serum (from SDPM1-immunized mice, at 1:500 dilution, or SDPM1-immune serum pre-cleared on Ap1–42 amyloid) for a period of time where amyloid formation was saturating (5 days for Aβ1–40, 5 hours for Aβ1–42) at 37°C, after which proteins were mixed with Thioflavin T and absorbance read at 490 nm (with excitation at 445 nm) using a SpectraMax M2 plate reader (Molecular Devices; Sunnyvale, CA). Time courses were performed to identify points of maximal amyloid formation in all instances, and these times were used for final comparisons. Values for Aβ peptide alone were used as the positive control, and values for SDPM1, SDPM2, or serum alone as background. Thioflavin T fluorescence measures for Aβ1–40 and Aβ1–42 peptides yielded signals at least 3 times buffer alone samples in all instances. To characterize Aβ1–42 oliogmers, a portion of the sample (for the final time point of 5 hours) was analyzed by separating Aβ oligomers on 10–20% SDS-PAGE Tris/Tricine gels. Aβ peptides were identified by immunoblotting for Aβ1–40/1–42 with a rabbit polyclonal antiserum and appropriate HRP-conjugated secondary antibody, as before(Kang et al., 2003).
Similar amyloid formation and trapping experiments were done using monoclonal antibodies instead of peptides to assess inhibition of amyloid formation. For amyloid formation, Aβ1–42 peptide was mixed with buffer alone (no antibody), P4D6, or P1C11 at 10µg/mL, prior to Thoflavin T assay. Antibodies were first purified from hybridoma supernatant using goat anti-mouse IgM agarose affinity columns, following the manufacturer’s protocol (American Qualex; San Clemente, CA). Purified antibody was dialyzed in TBS and concentrated, as needed, using a 10,000 MW spin column. Protein concentration was assessed using a modified Bradford assay (Pierce BCA Protein assay kit; Fisher Scientific 23227) coupled with conformation of relative purity by SDS-PAGE with silver staining (Pierce;24597), as before(Yoon et al., 2009). A fraction of these experiments was also analyzed by SDS-PAGE with subsequent immunoblotting for Aβ1–40/42 as above.
Antibody binding experiments
Antibodies were purified on anti-mouse IgM agarose, concentrated, and protein levels assessed as above (Amyloid formation and trapping assays). For binding to peptides, 25ng of streptavidin was immobilized overnight on 96-well ELISA plates in 50mM bicarbonate buffer, pH. 9.5. Plates were washed in TBS and blocked in TBS with 3mg/mL BSA. One hundred ng of biotin-SDPM1, biotin-SDPM2, or buffer alone were added in TBS/BSA for 2 hours at room temperature. After washing in TBS, antibodies were added at the indicated concentrations in TBS/BSA for one hour at room temperature, after which plates were extensively washed in TBS and HRP-conjugated secondary antibody was added for 1 hour at room temperature. After further washing, plates were developed as before(Martin et al., 2009). Antibody binding to Aβ1–40 or Aβ1–42 amyloid was done as described above (Peptide binding experiments), only antibodies were added instead of SDPM1 or SDPM2, followed by appropriate secondary reagents.
Western blotting
To blot for Aβ amyloid forms, samples were boiled in SDS with reducing agent, separated on 10–20% Tris-tricine polyacrylamide gels, transferred to nitrocellulose, blocked in TBS with 0.1% Tween 20 (TBST) containing 5% non-fat dry milk, and incubated with rabbit anti Aβ1–40/42 at 1:10,000 dilution (in TBST with 2.5% non-fat dry milk). After washing in TBST, goat-anti-rabbit IgG-HRP secondary antibody was added (diluted at 1:10,000 in TBST with 2.5% non-fat dry milk), after which blots were washed in TBST and developed using ECL chemiluminescence (Amersham, Piscataway NJ) as before(Martin et al., 2009). To identify Aβ amyloid in total brain lysate from APPswePSEN1(A246E) mice, 100µg of total (SDS-soluble) cell lysate was loaded per lane. Immunoblots for APP were done as described above, only anti-V5-HRP antibody (Invitrogen, R961) was added to recognize the GKPIPNLLGLDST C-terminal epitope tag on recombinant APP WT or APPswe protein. For this experiment, 40µg of whole cell lysate was loaded from transfected and control cells.
Transfection of cell lines and immunoprecipitation
African green monkey SV40-transformed kidney fibroblast (COS7) cells were grown in Dulbecco’s Modified Eagles Medium (DMEM) with 10% fetal calf serum, 50µg/mL streptomycin, and 50 U/mL penicillin. When cells reached 40% confluence, they were transfected with a cDNA encoding human amyloid precursor protein (APP, 695) that was wild type (WT) or contained the Swedish early onset AD mutations (APPswe). Both constructs contained a V5 peptide epitope tag (GKPIPNLLGLDST) at the C-terminus of the protein. Transfections were done using Effectene transfection reagent (Qiagen) according to the manufacturer’s instructions. After 48 hours, cells were washed in PBS and solubilized in buffer containing 1% Nonidet P40 (NP40), 0.1% sodium dodecyl sulfate (SDS), 0.5mM deoxycholate, and 5mM EDTA with protease inhibitors (Roche, complete, #11-873-580-002). After sonication and subsequent shaking for 20 minutes, cell lysates were collected and unsolubilized material separated by centrifugation at 16000g for 20 minutes. Protein amounts were measured using a modified Bradford assay (Pierce BCA Protein assay kit; Fisher Scientific 23227).
For P4D6 immunoprecipitation, 1mg of protein from NP40-extracted cell lysate was incubated with 10µg of P4D6, P1C5, or buffer alone overnight at 4°C. Samples were then incubated with 100µL of pre-washed goat-anti-mouse IgM agaorse (Qualex) with gentle shaking overnight at 4°C. Pellets were spun at 1000g for 5 minutes, washed repeatedly in PBS, and the pellet solubilized in Lammeli sample buffer with β-mercaptoethanol. After boiling, precipitated proteins were separated on 4–15% SDS-PAGE gels and blotted with anti-V5 or Aβ1–40/42 antibodies. For SDPM1 precipitation, the same 1mg of cell lysate was incubated with 50µg of biotin-SDPM1, biotin-SDPM2, or TBS overnight at 4°C. One hundred µL of streptavidin agarose (Pierce, 20347) was added the next day for 2 hours at 4°C. After extensive washing, pellets were solubilized as above, proteins separated by SDS-PAGE, and Aβ oligomers blotted using biotinlyated-6E10 monoclonal antibody, followed by HRP-streptavidin. For 6E10 immunoprecipitation, 1mg of NP40 cell lysate was incubated with 4µg of biotin-6E10 (Covance, SIG-39350) overnight, followed by incubation with 100µL of streptavidin agarose as above. After washing, precipitated protein was separated by SDS-PAGE and immunoblotted with P4D6.
Immunization protocol
SDPM1 or SDPM2 peptides conjugated with an N-terminal biotin were synthesized and purified (as in (Kang et al., 2003)) by Anaspec (San Jose, CA) to greater than 90% purity, determined by HPLC and mass spectrometry. Peptides were dissolved in DMSO at 50mg/ml and stored frozen at −80°C. Prior to immunization, peptides were thawed and diluted into Tris-buffered saline, pH 7.4 (TBS). Streptavidin (purified recombinant protein from Streptomyces avidinii produced in E. coli-Sigma; St. Louis, MO) was diluted in TBS at 250µg/ml and sterile-filtered through a 0.22µm membrane. Streptavidin solution was then conjugated (4:1) with biotinylated SDPM1 or SDPM2 peptide overnight at 4°C (100µg of SDPM1 or SDPM2 peptide with 25ug of streptavidin), after which it was warmed to room temperature and injected subcutaneously into APPswePSEN1(A246E) mice. Mice were injected once a month from 5 months of age until 12 months of age, for a total of 8 injections for the “Young” immunized mouse protocol or from 12 months of age until 18 months of age, for a total of 7 injections, for the “Old” immunized mouse protocol.
For mice immunized with adjuvant, peptide-biotin-streptavidin antigen was mixed 1:1 with adjuvant prior to injection, emulsified in a bath sonicator, and injected as above. For no peptide controls with adjuvant, and equivalent volume of sterile TBS was mixed with adjuvant, emulsified, and injected. Mice immunized with adjuvant were given a first immunization using TiterMax Gold (Sigma T2684). Subsequent injections were done using Freund’s incomplete adjuvant (Sigma F5506).
Determination of antibody titer and subtype
Mice were bled via nicking of the tail vein at various months post-immunization. No significance in titers was observed between the final two months of each experiment, suggesting titers had reached a plateau by the end of the experiment. Red blood cells were clotted for one hour at 37°C and serum separated as before(Nguyen et al., 2002). Serum was diluted in TBS and added to 96-well ELISA plates coated with streptavidin-peptide conjugates (at 100ng SDPM1- or SDPM2-biotin-peptide/25ng streptavidin per well) or with streptavidin alone. Streptavidin was first immobilized on plates in 50mM sodium bicarbonate, pH 9.5 overnight at 4°C, followed by incubation with biotinylated peptide overnight in TBS at 4°C. To determine total SDPM1-specific titer, plates were blocked in TBS with 3mg/ml BSA, incubated with serum diluted in TBS with 3 mg/ml BSA, washed, and incubated with goat anti-mouse IgG+IgM conjugated with horseradish peroxidase (Jackson Immunochemicals, Seattle WA) at 1:1000. After washing in TBS, plates were developed for peroxidase activity and measured on a plate reader as before(Kang et al., 2003). Signals from serum dilutions were subtracted from secondary antibody alone in all instances. To determine antibody subtypes of SDPM1-specific titers, the protocol was redone, only secondary antibody step was replaced by HRP-conjugated goat-anti-mouse antibodies specific for mouse IgM, IgG1, IgG2a, IgG2b, IgG3, or IgA (Southern Biotech:5300-05) Assay of titers against Aβ amyloid were done in a similar manner, only Aβ1–40 or Aβ1–42 amyloid was made by dilution of peptide in TBS for 7 days at 50µg/ml, after which amyloid was diluted to 100ng/well in 50mM sodium bicarbonate, pH 9.5 and plated on 96-well ELISA plates overnight.
Immunohistochemistry and immunofluorescence microscopy
For Young-immunized mice, without adjuvant, SDPM1-, SDPM2-, and Mock-immunized mice were exsanguinated by perfusion in PBS followed by perfusion and further fixation in 2% paraformaldehyde. For Young- or Old-immunized mice with adjuvant, brains were dissected following exsanguination in PBS, bisected at the midsagittal plane, and either snap-frozen or placed in 2% paraformaldehyde overnight. Fixed brain hemispheres were washed for 1 hour with 0.1M glycine, washed in PBS, and placed in 30% sucrose. Brains were sunk in 30% sucrose for 3 days at 4°C and frozen in OCT (Optimal Cutting Temperature) compound using dry ice-cooled isopentane. Ten µm sagittal sections of brain were cut on a cryostat. For Thioflavin S staining, sections were dipped in doubly distilled water (ddH2O) and stained with Mayer’s hematoxylin for 5 minutes. Slides were rinsed in distilled water for 2 minutes and stained in 1% Thioflavin S for 5 minutes, after which they were soaked in 70% ethanol for 5 minutes and rinsed in running distilled water. For Congo Red staining, slides were dipped in ddH2O and stained for 20 minutes with filtered Congo Red solution (0.5% Congo Red, 50% ethanol). Slides were rinsed in running ddH20 for 5 minutes, dipped in alkaline alcohol (1% NaOH, 50% ethanol), and counterstained with Mayer’s hematoxylin for 30 seconds. Slides were rinsed in running ddH20 for 2 minutes, dehydrated through 95% ethanol and then 100% ethanol for 3 minutes each, cleared in xylene for 3 minutes, and mounted in cytoseal. For Aβ1–40/1–42 immunohistochemical staining, sections were blocked in PBS with 10% goat serum, stained with anti Aβ1–40/Aβ1–42 polyclonal antibody (Chemicon; Temecula, CA), washed in PBS, stained with goat anti-rabbit secondary antibody conjugated with alkaline phosphatase, washed in PBS, and developed as before(Kang et al., 2003). Secondary antibody alone gave no signal using this method. For Aβ1–40/42 immunostaining, some sections were pretreated with 90% formic acid for 4 minutes to enhance antibody access to Aβ amyloid. Such slides were not used for quantitation, but yielded similar trends in results. Immunofluorescence staining for amyloid precursor protein APP (both N-terminal (gift from Chris Phiel, NCH), C-terminal (Sigma; St. Louis, MO), Ionized calcium binding adaptor molecule 1 (Iba1) (Wako;Richmond, VA), Cd68 (AbD serotec;Oxford, UK), glial fibrillary acidic protein (GFAP) (EnCor Biotech;Gainesville, FL), CD4, CD8, and B220 (all BD Biosciences;San Jose, CA) were done as before. Sections were blocked for 2 hours in 10% goat serum at room temperature and incubated with primary antibody overnight at 4°C. Sections were then washed with PBS and incubated in goat anti-rabbit IgG or goat anti-rat IgG conjugated to Cy3 for 1 hour at room temperature. Sections were washed with PBS, dried, and mounted using glycerin jelly medium. In addition, some sections were also stained with P4D6 or streptavidin. Here, brain sections were blocked for 2 hours in 5% BSA and 5% goat serum at room temperature and incubated overnight with P4D6. Sections were washed with PBS before incubating with secondary antibody anti-rabbit IgM conjugated to Cy3 for 1 hour at room temperature. For streptavidin staining, sections were incubated with Cy3-conjugated streptavidin directly. Sections were washed with PBS, dried, and mounted using glycerin jelly medium. For Young (no adjuvant) brain staining of Aβ1–40/42, HRP conjugated goat anti-rabbit secondary antibody was added, followed by color HRP staining, as before(Kang et al., 2003). In all cases, species-appropriate Cy3-conjugated secondary antibodies were used to identify immunostaining (Jackson Immunochemicals;Seattle, WA) and addition of secondary antibody alone was done to identify non-specific background staining.
Image analysis
Ten µm serial sagittal sections were collected from fixed brain hemispheres beginning 720µm laterally from the midplane by cryostat sectioning for staining of fibrillar Aβ amyloid (with Thioflavin S) to assess plaque burden, plaque number, and plaque size. Eight to twelve sagittal brain sections were used, each spaced 80µm apart, for imaging of amyloid plaques or immunostaining. Staining images were recorded using a Zeiss axiophot epifluorescence microscope and AxioVision LE 4.1 software using a 10X (for Thioflavin S and Aβ1–40/42 staining) or 20X (for GFAP, CD68, and Iba1) objective. All images were collected at the same exposure settings and using the same illumination intensity and filters. Images were converted to TIFF format, converted to grayscale, analyzed using NIH Image J (1.42) software as previously described(Head et al., 2001). Sections were blinded with respect to the investigator prior to analysis, and multiple investigators participated in analysis of each experiment. Thioflavin S staining of fibrillar Aβ amyloid plaques was used to determine amyloid plaque burden and number. Sections were quantitated for staining pixel density, number of plaques (greater than 10µm2), and for plaque area using full sections of hippocampus or equivalent numbers of sections from frontal, medial, and temporal cortical regions (8–12 each), which were averaged together to yield data for cortex. Each image analyzed was 708×528µm in total area (for Thioflavin S and Aβ1–40/42) or 354µm×265µm in total area (for GFAP, Iba1, and CD68). The threshold for detection of staining was determined and held constant throughout image analysis. Thioflavin S-positive plaques were counted by visual inspection of all stained sections, blinded with respect to treatment condition. Amyloid plaque burden was expressed as the percentage area of plaque staining relative to total image area containing brain tissue. Staining of blood vessels (confirmed by counterstaining with hematoxylin/eosin) was discounted, though this too was reduced by SDPM1 immunization. Quantitation of total staining signal, plaque burden, and plaque number was determined independently by at least two investigators with the same results. Aβ1–40/42, GFAP, CD68, and Iba1 immunostaining was assessed via similar methods, but individually stained cells were also quantified in addition to semi-quantitative measures of total staining. Hematoxylin counterstaining was done to confirm brain structures. Analysis of GFAP-, Iba1-, CD68-, CD3-, CD4-, CD8-, and B220-positive cells were done after immunofluorescence staining of brain sections. Random 20x fields were surveyed from sagittal sections of brain taken 200–300µm from the midline. B220-, CD3-, CD4-, and CD8-positive cells were only found within the vasculature and not in brain parenchyma, while the converse was true for Iba1, GFAP, and CD68.
Measurement of Aβ1–40 and Aβ1–42 peptide levels
Snap-frozen brain regions (cortex, hippocampus, and cerebellum) were dissected and weighed. Soluble fraction was extracted in Tris-buffered saline (TBS) containing protease inhibitors (Roche, complete #11-873-580-002) and 2% SDS. Insoluble fraction was precipitated at 16000g for 20 minutes and the pellet re-extracted with 70% formic acid. After sonication, formic acid extracted samples were centrifuged (at 100,000g for one hour at 4°C) and the supernatant neutralized in 1M Tris-base/0.5M NaH2PO4. Soluble fraction and (neutralized) insoluble fractions were diluted in standard diluent buffer and assayed using immunoassay kits specific for human Aβ1–40 (Invitrogen, KHB3482) and for human Aβ1–42 (Invitrogen, KHB3442) following the manufacturer’s instructions. ELISA plates were measured for absorbance at 450nm on a SpectrMax M2 plate reader and concentrations of Aβ1–40 and Aβ1–42 calculated based on standard curves of known peptide run at the same time. Total peptide amount was then normalized to the wet weight of the dissected brain region in the original homogenate, and final values reported as micrograms of peptide per gram wet brain weight.
Morris Water Maze Tests
Groups of 12-month and 18-month old APPswePSEN1(A246E) mice treated with SPDM-1, SPDM-2 or PBS and age-matched wild type (WT) controls were tested in the Morris water maze. The task was conducted in a large pool (diameter 122 cm) filled with water (24°C) made opaque using non-toxic white paint. A hidden platform (square, 10 cm2) was placed 1 cm beneath the surface of the water in the E quadrant of the pool. Visual cues were mounted on a screen surrounding the pool. The mice were first pre-trained for 1 day to find and climb on to the hidden platform within 60 s after being placed in the water. If a mouse was unable to find the platform, it was placed there manually for 10 s by the experimenter. Mice were given up to three times to find the platform; mice unable to find the platform in the course of the three trials were excluded from the experiment. Mice were tested for eight consecutive days with four trials per day, each of 60 sec. The platform location was kept constant and the starting position was varied randomly between five different locations surrounding the platform. Mice were placed in the water with their nose pointing towards the wall at one of the starting points. They were given 60 s to find and climb on to the platform, or were placed there for 10 sec by the experimenter. Mice were picked up the tail, dried off, put in their cages and were given 5–15 min rest between trials. Swim speed (cm/s), latency (s), and distance swum (cm) were recorded by SMART video-tracking system. A probe test to assess memory was conducted two hours after the last trial. The hidden platform was removed and the mice were placed in the water and given 60 s to swim as usual. Percentage of time spent in the quadrant of the location of the hidden platform was recorded.
Open Field Tests
Ambulatory movements and rearing events were determined using the Photobeam Activity System (PAS, San Diego Instruments) open field test. All measurements were done in mouse behavior rooms at the same time of day with defined temperature, ambient noise, and ambient light. Each mouse was placed individually in a 16×16 inch clear acrylic chamber with photobeams that run along its base at one inch spacing along the X- and Y-axis. Ambulatory activity was recorded every time the animal crossed from one square inch region to another. Peripheral movement reflects beam crossing in the outermost 1 square inch squares of the cage on any side, while center movement reflects all other cage regions. Movements were recorded in 5 4-minute sessions. Total recorded events for the entire 20 minutes were summed for each independent measure, with 6 total measures being taken. Both ambulatory and fine movements were recorded. Ambulatory movements were defined as movements in which an animal crossed a single photobeam. Fine movements were recorded as photobeams crossed multiple times in sequence. Rearing events were also recorded, using software that specifically defines this pattern of behavior.
ELISPOT assays
Spleens were dissected and splenocytes made by tituration in RPMI 1640 media and filtration through a cell strainer. Cells were then collected by gentle centrifugation (1000g). After further purification, splenocytes were cultured at 4×105 cells/well and stimulated with 2µg/mL SDPM1 or Aβ1–42, control buffer, or 10µg/mL Concanavalin A (ConA) in the presence of tissue culture media (RPMI 1640 with 10% FCS and P/S) for 48 hours. Plates were washed in PBS 6 times and subjected to ELISPOT assays for Interleukin 4 (IL4), to detect Th2-type responses (U-CyTech CT319-PB5) or Interferon gamma (IFNγ), to detect Th1-type responses (U-CyTech, CT317-PB5), according to the manufacturer’s instructions. Spots per well were counted manually after imaging of plates on a Zeiss bright field microscope using Zeiss imaging software.
T cell proliferation assays
Analysis of T cell proliferation was performed using splenocyte cultures from individual animals. Splenocytes were isolated from Mock- and SDPM1-immunized APPswePSEN1(A246E) animals that were immunized with adjuvant beginning at 5 months of age and analyzed at 12 months of age (Young protocol). At the time of sacrifice, the spleen was dissected and splenocytes isolated as above (ELISPOT assays). For this assay, 6×105 isolated splenocytes were cultured per well (in 96-well ELISA plates) and incubated with control buffer or 10µg/ml SDPM1, SPDM2, Aβ1–42, Concanavalin A (ConA) for 48 hours in RPMI media. After 48 hours stimulation, 2µCi of 3H thymidine (TRK 424, GE Healthcare) was added for an additional 24 hours. Cells were subsequently washed and then lysed using a Wallac harvester and 3H measured using a scintillation counter. Stimulation index (SI) was determined as previously described(Kutzler et al., 2006).
Statistics
For comparisons between only two groups, significance was determined using a two-tailed unpaired Student’s t test. For comparisons with more than two groups, significance was determined by ANOVA with post-hoc t test (GraphPad Prism 4.03 software, GraphPad Software, San Diego, CA).
Results
SDPM1 and SDPM1 peptide mimotope antibodies bind low molecular weight Aβ1–40 and Aβ1–42 amyloid and block subsequent Aβ amyloid aggregation
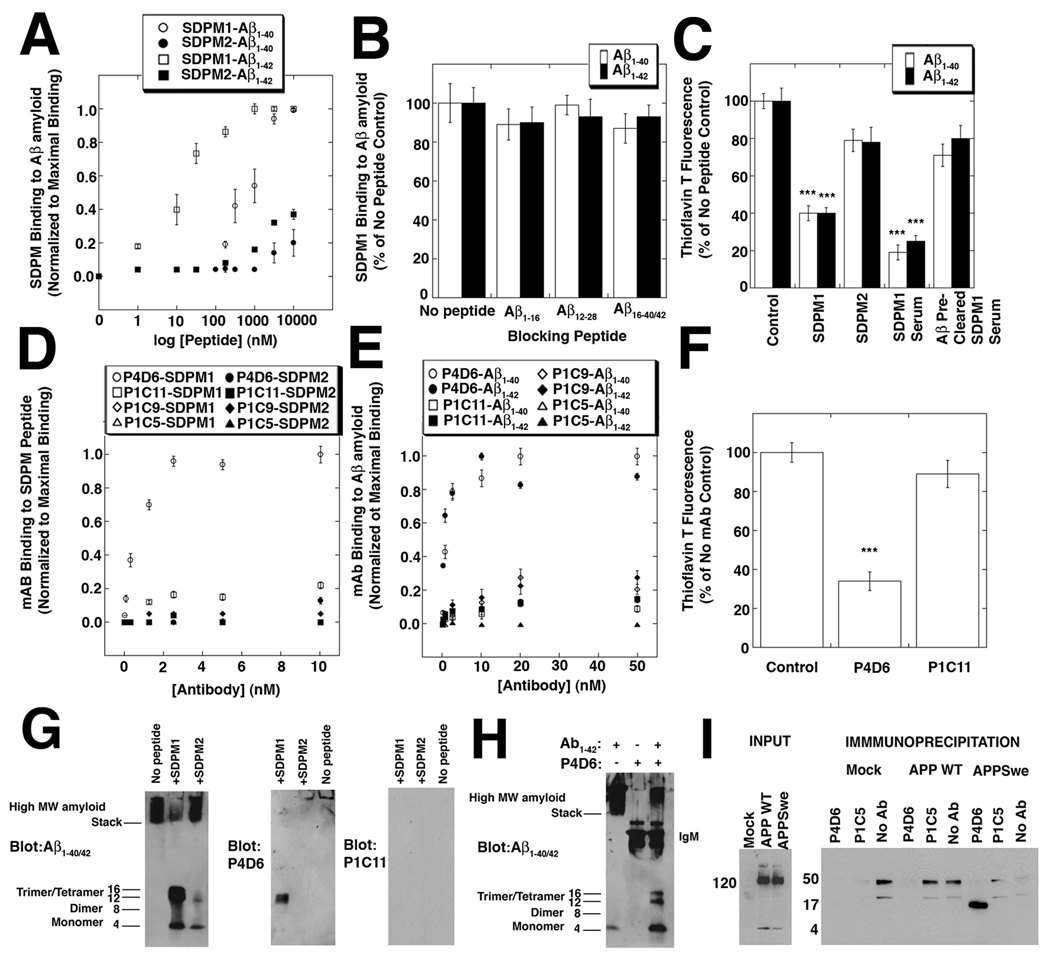
We had identified several candidates that could be used in a peptide mimotope vaccine strategy for Alzheimer’s disease (AD)(Kang et al., 2003). Using a cysteine-bounded phage peptide display library with 5×107 random 20 amino acid insertions, we identified two peptide sequences that specifically bound Aβ1–40 amyloid, but not the linear peptide, with high affinity(Kang et al., 2003). These same peptides, when synthesized chemically, also specifically bound Aβ1–40 amyloid in vitro and amyloid plaques in AD brain(Kang et al., 2003). The SDPM1 peptide (AEC-DWGKGGRWRLWPGASGKTEA-CGP), which was most efficacious in binding amyloid plaques in AD brain(Kang et al., 2003), is used here. The presence of a cysteine at each end of the SDPM1 peptide, a configuration present in all peptides used in the original phage library screened, was required for high affinity binding to Aβ1–40 amyloid, with half maximal binding occurring in the 1µM (Fig 1A). A peptide containing the same 20-amino acid peptide sequence, but without the terminal cysteines (SDPM2; DWGKGGRWRLWPGASGKTEA), showed minimal binding to Aβ1–40 amyloid in the same concentration range. Though SDPM1 was isolated in a screen for Aβ1–40 amyloid binding peptides(Kang et al., 2003), it bound to Aβ1–42 amyloid with even greater affinity, showing half maximal binding of 20nM (Fig. 1A). Binding of SDPM2, by contrast, was only significant for Aβ1–42 amyloid at concentrations above 1 µM. Binding of SDPM1 was specific for Aβ1–40 or Aβ1–42 amyloid and could not be blocked by a molar excess of linear Aβ1–16, Aβ12–28, or Aβ16–40 or Aβ16–42 peptide (Fig. 1B). These linear peptides comprise overlapping regions of the Aβ1–40 and Aβ1–42 sequence but do not form amyloid over the time course of this experiment. Thus, SDPM1 binding is specific for amyloid forms of Aβ1–40 and Aβ1–42.
Figure 1. SDPM1, and SDPM1-mimotope antibodies bind low molecular weight Aβ1–40/1–42 amyloid and block subsequent Aβ amyloid aggregation.
(A) Binding of the SDPM1 and the SDPM2 peptide to Aβ1–40 and Aβ1–42 amyloid. (B) Addition of molar excess of monomeric Aβ1–16, Aβ12–28, Aβ16–40, or Aβ16–42 peptide does not block SDPM1 binding to Aβ1–40 or Aβ1–42 amyloid. (C) Addition of SDPM1 or SDPM1 immune serum with monomeric Aβ1–40 or Aβ1–42 amyloid blocks aggregation of Aβ amyloid. Pre-clearing of SDPM1-immune serum on Aβ1–42 amyloid removes its blocking ability. (D) P4D6 binds SDPM1 (open circles) but not SDPM2 (closed circles), while P1C11, P1C9, and P1C5 do not bind either SDPM1 or SDPM2 with high affinity. (E) P4D6 shows high affinity binding to Aβ1–40 (open circles) and Aβ1–42 (closed circles) amyloid, while P1C11, P1C9, and P1C5 do not. (F) P4D6 blocks amyloid aggregation of Aβ1–42 peptide in aqueous solution, while P1C11 does not. (G) Addition of SDPM1 to Aβ1–42 peptide blocks formation of high molecular weight (MW) amyloid formation and traps 12 kDa (trimer) and 16 kDa (tetramer) forms. These forms are blotted by P4D6 but not by P1C11. (H) Addition of P4D6 with Aβ1–42 peptide blocks high MW amyloid formation and traps trimer/tetramer Aβ amyoid forms. (I) COS7 cells were either mock-transfected or transfected with a V5-tagged cDNA for amyloid precursor protein (APP WT) or the Swedish early onset AD mutant (APPswe). Input from whole cell lysates, blotted with a V5 antibody, showed equivalent levels of APP WT and APPswe were present. Equivalent amounts of non-ionic detergent cell lysates were precipitated with resin alone (No Ab), P4D6, or P1C5, then blotted with antibody to Aβ1–42. Only P4D6 precipitated low molecular weight (16kDa) Aβ amyoid. Errors are SEM for n=4 (A, B), 6 (D–E), or 3 (C, F) measurements per condition.
Because SDPM1 was isolated as binding to very low concentrations of Aβ1–40 amyloid(Kang et al., 2003), we surmised that SDPM1 could represent a binding epitope present in relatively low density Aβ oligomers and as such might be able to block subsequent Aβ aggregation into larger molecular weight (MW) oligomeric forms. To test this, we compared the ability of SDPM1 and SDPM2 to block the ability of monomeric Aβ1–40 and Aβ1–42 peptides to form Aβ amyloid after addition in aqueous solution. This was determined by measuring increased Thioflavin T fluorescence, a measure of increased secondary structure formation as monomeric peptides aggregate to form Aβ amyloid. SDPM1 inhibited amyloid aggregation for either Aβ1–40 or Aβ1–42 peptide by over 60% (Fig. 1C). SDPM2, by contrast, showed no significant decrease in Thioflavin T signal (though it did trend downward). Addition of SDPM1 alone did not lead to development of increased Thioflavin T signal over time, suggesting it does not form amyloid when added alone. We next tested whether SPDM1 would induce peptide mimotope antibodies that would be similarly effective at blocking Aβ amyloid aggregation. To do this, we immunized mice with SDPM1, presented with adjuvant as a tetrameric peptide coupled to streptavidin (via an N-terminal biotin peptide linker). Immunized mice showed high SDPM1 antibody titers. Detection of titers with an OD greater than 0.1 to SDPM1 (subtracted for streptavidin) ranged from 1 in 6,400 to 1 in 25,600 in five different immunized animals, while control immunized animals never showed SDPM1-specific titers (not shown). Serum from SDPM1-immunized animals was even more effective (70–80% inhibition) than the SDPM1 peptide at reducing Aβ amyloid aggregation (Fig. 1C). If SDPM1-specific serum was pre-cleared against Aβ1–42 amyloid prior to the experiment, the amyloid blocking response was eliminated (Fig. 1C). Serum from mice not immunized with SDPM1, when used at similar dilutions, showed no significant effect (not shown).
To show that SDPM1 induced the production of monoclonal antibodies that specifically recognize SDPM1 and block amyloid aggregation, we made hybridomas from the spleens of SDPM1-immunized mice. We identified several monoclonal antibodies that had specific high affinity binding for SDPM1 (compared to SDPM2 and streptavidin, Fig. 1D). Additionally, we isolated antibodies that either bound both SDPM1 and SDPM2 equally well or that bound only to streptavidin, which we used to conjugate the biotinylated SDPM peptides for immunization. We chose one SDPM1-specific antibody, P4D6, and three control antibodies, P1C11, P1C5, and P1C9, all IgMs, for subsequent experiments (Fig. 1D). Like SDPM1 peptide, P4D6 antibody bound with high affinity to Aβ1–40 and Aβ1–42 amyloid (Fig. 1E), with half maximal binding to both forms occurring at 2nM. Like SPDM1, P4D6 also blocked Aβ amyloid aggregation in the Thioflavin T assay when mixed with either monomeric Aβ1–40 or Aβ1–42 peptide, while control monoclonal antibodies did not (Fig. 1F). Thus, immunization with SDPM1 led to the production of monoclonal antibodies that had the same activity in binding Aβ amyloid and in blocking subsequent Aβ amyloid aggregation.
Since SDPM1 does not bind monovalent Aβ1–40 or Aβ1–42 peptide(Kang et al., 2003), we wished to determine the oligomeric forms of Aβ amyloid the peptide bound to. To do this, we first mixed SDPM1 with monovalent Aβ1–42 in Tris-buffered saline (TBS) to induce amyloid formation and trap SDPM1-bound Aβ amyloid species. We then separated the isolated Aβ amyloid forms on gradient SDS-PAGE gels and identified them by immunoblotting with a polyclonal antibody to Aβ1–40/42. Addition of SDPM1 reduced formation of high molecular weight (MW) Aβ aggregates, which run in the stacking region of such gels, and trapped 12–16kDa forms that correspond to Aβ trimers and tetramers (Aβ1–42 is 4kDa as a monomer) (Fig. 1G). As before, SDPM2 had no effect on Aβ amyloid formation in vitro (showing no difference from control with no peptide added). P4D6 also recognized trimer/tetramer Aβ amyloid forms by immunoblotting, while control antibodies (e.g. P1C11) did not (Fig. 1G). Addition of P4D6 with monomeric Aβ1–42 peptide led to an analogous decrease in high MW Aβ amyloid and a trapping of 12–16 kDa Aβ forms (Fig. 1H). Thus, SDPM1 and an SDPM1-specific antibody (P4D6) both block Aβ amyloid formation (Figs. 1A and 1D), most likely by binding to trimer or tetramer Aβ amyloid species. Next, we showed that P4D6 specifically precipitated low molecular weight (presumed tetramer) Aβ amyloid from COS7 cells transfected with APPswe, a cDNA encoding the Swedish early onset AD mutations in amyloid precursor protein (APP), while it did not precipitate any APP protein from cells similarly transfected with wild type APP (APP WT, which does not produce significant Aβ1–40/42 peptide) (Fig. 1I). Precipitation of the same lysates with SDPM1 peptide yielded similar results, with tetramer Aβ amyloid precipitated from APPswe-transfected cells (S1A). Similarly, P4D6 blotted tetrameric (16kDa) Aβ amyloid aggregates from APPSwe (but not APP WT)-transfected cell lysates precipitated with 6E10, an Aβ1–40/42 monoclonal antibody (S1B).
Immunization with SDPM1 increases anti-SDPM1 and anti-Aβ amyloid antibodies and lowers amyloid plaque burden in APPswePSEN1(A246E) mice
As we had found that SDPM1 could induce antibodies that block Aβ amyloid aggregation, we next tested whether immunization of APPswePSEN1(A246E) transgenic mice, a mouse model for Alzheimer’s disease(Borchelt et al., 1997), with SDPM1 would block amyloid formation in vivo. Immunization with an equivalent amount of SDPM2, which does not block Aβ aggregation, or immunization with no peptide, was done as a control. As peptides require a multivalent presentation to elicit significant immune responses, we synthesized SDPM1 and SDPM2 with an N-terminal biotin and bound these peptides in a 4:1 ratio to streptavidin (which binds four biotins per molecule) to create the immunogen. We performed three immunization experiments. First, we immunized relatively young APPswePSEN1(A246E) mice subcutaneously once a month with SDPM1, SDPM2, or no peptide from 5 months of age (an age prior to significant amyloid plaque formation)(Borchelt et al., 1997) until 12 months of age (when plaque burden has become significant)(Borchelt et al., 1997). For this first experiment, we did not include adjuvant in the immunization schedule. For the second experiment, we immunized subcutaneously once a month, as before, only adjuvant was included to boost antibody titers. Complete adjuvant (TiterMax Gold, Sigma) was used for the first immunization, followed by Freund’s incomplete adjuvant for all subsequent immunizations. For the third experiment, we immunized relatively old APPswePSEN1(A246E) mice, after plaque burden was already evident(Borchelt et al., 1997). Here, we immunized 12 month-old APPswePSEN1(A246E) animals (with adjuvant), as before, once a month until the animals were 18 months old.
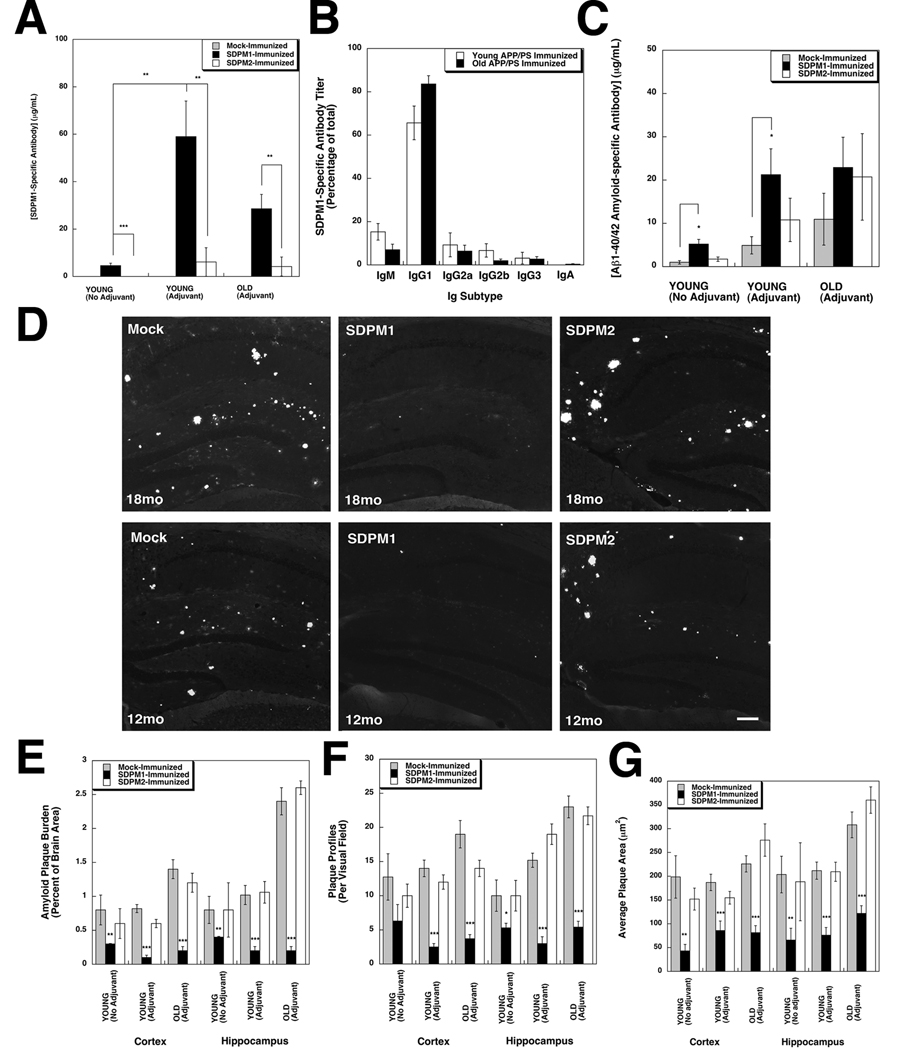
All mice were bled and titers against SDPM1 and SDPM2 peptide were measured (Fig. 2A and S2A). By the end of the experiment, young mice immunized without adjuvant had developed antibody titers to both SDPM1 and SDPM2 (ranging from 2–15µg/mL) (S2A). SDPM1-immunized mice showed increased SDPM1 titers relative to SDPM2, while SDPM2-immunized mice showed the opposite trend (S2A). Because SDPM1 requires cysteines at its ends to significantly bind Aβ amyloid, we subtracted the non-specific SDPM2 titer from the SDPM1 titer to determine the titer of relevant SDPM1-specific antibodies (Fig. 2A). For young mice immunized without adjuvant, SDPM1 immunization led to an SDPM1-specific antibody titer, on average, of 5±1µg/mL. As expected, young mice immunized with adjuvant showed increased titers to SDPM1 and SDPM2 (S2A). Here, SDPM1-immunized mice showed an average SDPM1-specific titer of 59±15µg/mL, a significant increase relative to mice immunized without adjuvant (Fig. 2A). Likewise, old mice (18mo) immunized monthly from 12mo onward with adjuvant showed an average SDPM1-specific titer of 29±6µg/mL. Mock-immunized and SDPM2-immunized mice never showed SDPM1-specific antibody titers under any conditions (Fig. 2A). With adjuvant, the predominant antibody subtype resulting from SDPM1 vaccination in both young and old mice was IgG1, with far more modest titers of IgM, IgG2a and IgG2b also being present (Fig. 2B). The 10:1 ratio of IgG1 to IgG2a suggests a predominant Th2 type immune response(Finkelman et al., 1990). Because SDPM1 can produce SDPM1-specific antibodies, such as P4D6, that also have high affinity for Aβ1–40/1–42 amyloid (Fig. 1D and E), we next assessed immune titers to Aβ1–40 and Aβ1–42 amyloid in SDPM-peptide-immunized and control mice (Fig. 2C). Unlike titers to the SDPM1 and SDPM2 peptides, APPswePSEN1(A246E) mice have a background level of antibody titer to Aβ amyloid, and this was increased in old animals relative to young ones (Fig. 2C). Nevertheless, young mice immunized with SDPM1, either with or without adjuvant, showed a significant increase in Aβ amyloid antibody titer relative to mock immunized animals (Fig. 2C); young SDPM1-immunized mice without adjuvant had Aβ amyloid titers of 5±1µg/mL (relative to 1±1 µg/mL for Mock) and young mice immunized with adjuvant animals had Aβ amyloid titers of 21±7µg/mL (compared to 5±2µg/mL for Mock). Old SDPM1-immunized mice also showed elevated Aβ1–40/42 titers compared to Mock-immunized controls (23±7µg/mL versus 11±6µg/mL), but this level of elevation did not reach statistical significance. While these anti-Aβ amyloid antibody levels would be considered modest were Aβ amyloid used as the immunogen, our in vitro studies suggest that SDPM1 vaccination would yield antibodies only to low molecular weight forms of Aβ amyloid (Fig. 1G–I), and such forms likely comprise a minority of Aβ amyloid’s total immunogenic repertoire.
Figure 2. Immunization with SDPM1 lowers amyloid plaque burden in APPswePSEN1(A246E) transgenic mice.
(A) SDPM1-specific antibody titers (total SDPM1 titer minus total SDPM2 titer) in APPswePSEN1(A246E) mice immunized once a month with SDPM1, SDPM2, or no peptide (Mock) beginning at 5 months of age and analyzed at 12 months of age (YOUNG), with or without adjuvant, or immunized beginning at 12 months of age and analyzed at 18 months of age (OLD) with adjuvant. (B) Breakdown of SDPM1-specific antibodies by subtype in SDPM1-immunized APPswePSEN1(A246E) mice (with adjuvant). (C) Antibody titers to Aβ1–42 amyloid for experiments described in A. (D) Hippocampus stained with Thioflavin S to visualize fibrillar Aβ amyloid in Young (12mo) and Old (18mo) immunized mice (with adjuvant) from A. Bar is 100µm. (E) Quantification of Aβ amyloid plaque burden as a percentage of total brain area in cortex and hippocampus. (F) Quantification of number of plaque profiles per unit area. (G) Quantification of average area per individual plaque. Errors are SEM for n=7–12 animals per condition, with n= 5–6 measurements per animal in A–C and 10–24 measurements per animal in E–G.
After immunization schedules were complete, we assessed amyloid plaque burden (Fig. 2D–G, S2B, S3–S4) in the hippocampus and cortex of APPswePSEN1(A246E) mouse brains. To do this, we quantified Thioflavin S staining of serial sagittal sections of brains from mock-immunized, SDPM1-immunized, and SDPM2-immunized mice, comparing mice immunized from a young age, with or without adjuvant, and mice immunized from an old age with adjuvant (Fig. 2E–G). Staining of fibrillar amyloid was evident in all mock-immunized and SDPM2-immunized hippocampus (Fig. 2D, S4–5) and cortex (S3–4), while staining in SDPM1-immunized brains was dramatically reduced (Fig. 2D, S3–4). Similar results were seen with Congo Red staining (not shown). In addition to a reduction in amyloid plaque burden (Fig. 2E), the overall number of plaques (Fig. 2F) and average area of each individual plaque (Fig. 2G), as well as the total pixels of Thioflavin S staining (S2C), were reduced in SDPM1-immunized cortex and hippocampus. Generally, young mice immunized with SDPM1 containing adjuvant had the lowest plaque burden (12±3% of mock-immunized in cortex and 20±4% of mock-immunized in hippocampus), while plaque burden in young mice immunized without adjuvant was higher (38±5% of mock-immunized in cortex and 50±7% of mock-immunized in hippocampus). As with Aβ immunization, mice immunized at an old age, where plaque burden was highest, also showed highly significant reductions in amyloid plaque burden, plaque number, plaque area, and Thioflavin S staining (Fig. 2E–G, Fig. S2B). Immunization with SDPM2, by contrast with SDPM1, showed no significant changes relative to mock-immunized control animals for any of these measures (Fig 2E–G, Fig S2B). Thus, immunization with SDPM1 reduces amyloid plaque burden in the brains of APPswePSEN1(A246E) mice, both when immunization is done prior to the time of amyloid formation and when immunization is done after significant amyloid formation has already occurred. Immunostaining for the biotinylated SDPM peptides (using streptavidin) or SDPM1 protein (using P4D6) showed no evidence for migration of for SDPM1 peptide from its subcutaneous injection site into the brains of SDPM1-immunized animals (not shown).
Immunization with SDPM1 decreases Aβ1–40 and Aβ1–42 peptide levels in APPswePSEN1(A246E) mouse brain
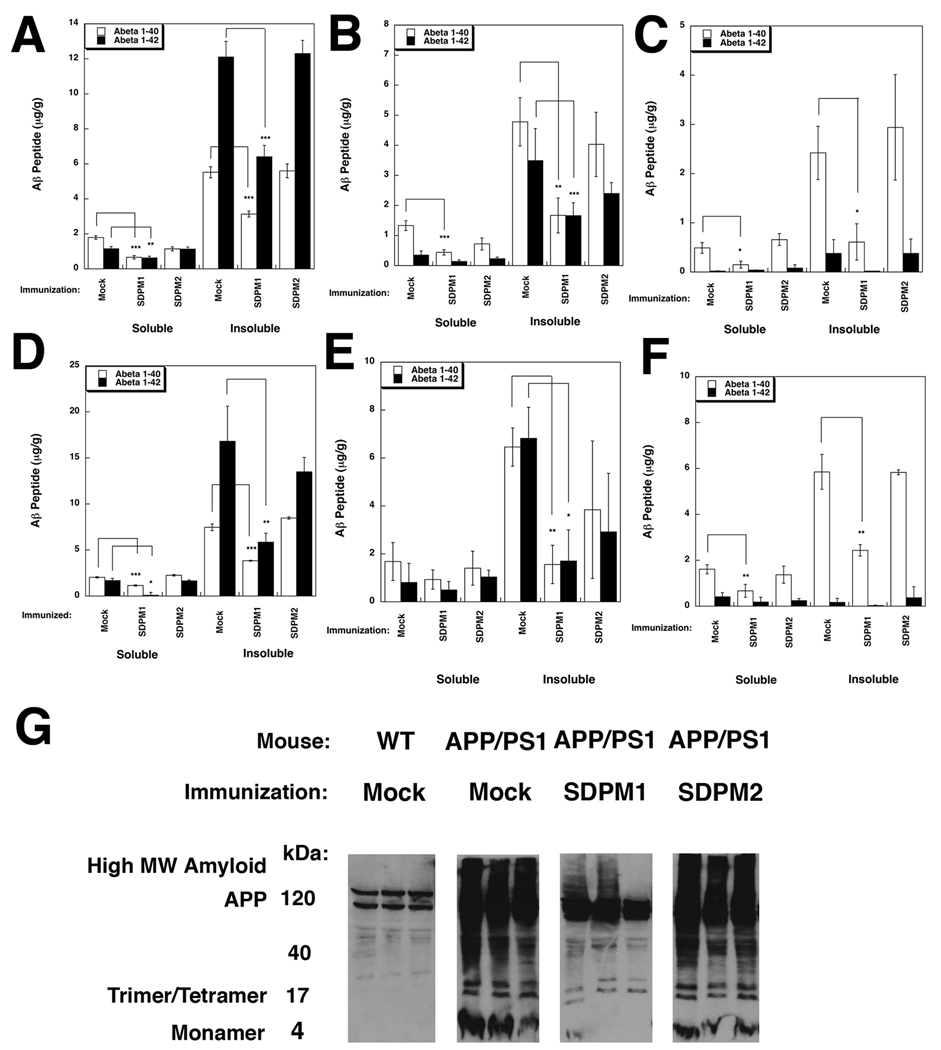
We next assessed Aβ1–40 and Aβ1–42 peptide expression in treated and mock-treated APPswePSEN1(A246E) brain. Immunostaining for Aβ1–40/1–42 was abundant in amyloid plaques of APPswePSEN1(A246E) brain but was generally reduced in the hippocampus (S5 and S7) and cortex (S6 and S7) of SDPM1-immunized mice. Measurements of Aβ1–40/42 immunostaining (using NIH Image J (V1.42) software) showed signal for SDPM1-immunized mice that was 25±6% of control in cortex and 14±3% of control in hippocampus (for young mice immunized with adjuvant). Aβ1–40/42 staining signal was also reduced in old mice immunized with SDPM1 (S2C). By contrast, Aβ staining levels in SDPM2-immunized mice was not significantly reduced, though it trended downward compared to mock-immunized animals (S2C). We also measured Aβ1–40 and Aβ1–42 peptide levels directly in dissected cortex, hippocampus, and cerebellum. We extracted SDS-soluble and -insoluble protein fractions and subjected them either to indirect Aβ1–40 or Aβ1–42 ELISA measurements (Figure 3A–F) or to Aβ1–40/42 immunoblotting after separation by SDS-PAGE (Fig. 3G). Young SDPM1-immunized APPswePSEN1(A246E) mice showed a significant reduction in both soluble and insoluble Aβ1–40 and Aβ1–42 in cortex (Fig. 3A) and hippocampus (Fig. 3B). Aβ1–40 levels were also significantly reduced by SDPM1 immunization in the cerebellum (Fig. 3C). Aβ1–42 levels were quite low in cerebellum, consistent with the low to absent amyloid plaque burden in this brain region of APPswePSEN1(A246E) animals(Borchelt et al., 1997). In all instances, the level of reduction in Aβ1–40/1–42 peptide levels resulting from SDPM1-immunization was greater than 50%. Results were similar for old mice immunized with SDPM1 (Figs. 3D–F), with the level of reduction again exceeding 50% in most instances (and for Aβ1–42 reaching a 65–75% reduction in the insoluble fraction for hippocampus and cortex). Aβ immunoblotting of the soluble fraction from cortex showed all APPswePSEN1(A246E) animals had equivalently high levels of APP transgenic protein but that SDPM1-immunized mice had large reductions in both high and low MW Aβ amyloid species (Fig. 3G).
Figure 3. Immunization with SDPM1 lowers brain levels of Aβ1–40 and Aβ1–42 in APPswePSEN1(A246E) transgenic mice.
(A–E) Aβ1–40 and Aβ1–42 levels were measured in Young (A–C) or Old (D–F) Mock-, SDPM1-, and SDPM2-immunized mice (with adjuvant) in cortex (A, D), hippocampus (B, E), or cerebellum (C, F). (G) Immunoblot with an Aβ1–42-specific polyclonal antiserum of 100µg of total SDS-soluble proteins isolated from Mock-immunized wild type (WT) or from Mock-, SDPM1- or SDPM2-immunized APPswePSEN1(A246E) cortex (APP/PS1). Examples from three different animals are shown per condition. Errors are SEM for n=7–12 animals per condition for n=3 measurements per animal per condition in A–F.
Immunization of APPswePSEN1(A246E) mice with SDPM1 prior to Aβ plaque formation inhibits loss of cognitive function in Morris Water Maze tests, but immunization after plaque burden is already significant does not
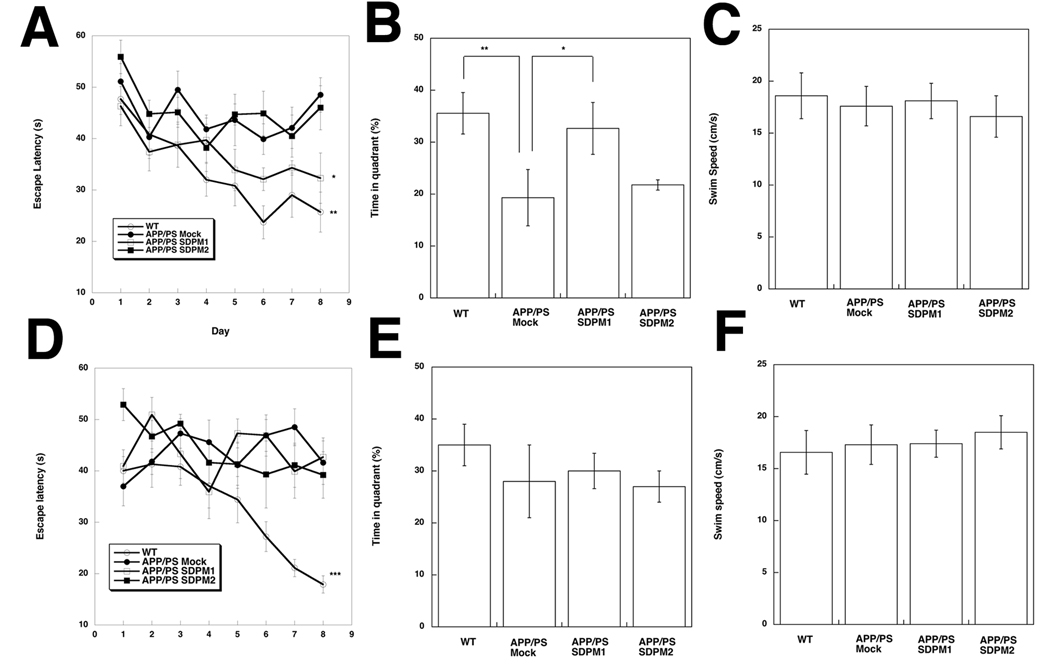
We next analyzed whether SDPM1 immunization led to improvement in cognitive function using Morris water maze tests. A comparison of age-matched wild type animals and mock-immunized APPswePSEN1(A246E) animals at 12 months of age (young) and at 18 months of age (old) showed that in both instances wild type (WT) animals learned to identify the target, showing reduced escape latency with repeated training (Fig. 4A and 4D). After 8 days of a daily training regimen, WT animals reduced the escape latency time to target by 50% for both experiments (young and old), while APPswePSEN1(A246E) animals showed no reduction in either instance. For young APPswePSEN1(A246E) animals (5mo start to 12mo end, with adjuvant), SDPM1-immunization reduced escape latency time to a level nearing wild type learning, and this was significantly different from mock-immunized APPswePSEN1(A246E) controls (Fig. 4A). After training, mice were also subjected to a probe test to measure memory of the learned skill. In this test, the time measured in the quadrant containing the platform the mice have been trained to find is measured in the platform’s absence. For young mice, WT animals spent significantly more time in the appropriate quadrant than mock-immunized APPswePSEN1(A246E) mice. Again, SDPM1-immunized APPswePSEN1(A246E) mice improved significantly compared to controls, spending almost the same amount of time in the appropriate quadrant as WT animals (Fig. 4B). By contrast, SDPM1-immunized animals that underwent immunization after amyloid plaque formation was already significant (old animal protocol, immunized starting at 12 mo and ending at 18mo) showed no improvement in either escape latency (Fig. 4D) or probe test (Fig. 4E). Thus, cognitive improvement resulting from immunization with SDPM1 may require immunization to occur at a young age, prior to significant Aβ amyloid formation. This was true despite the fact that Aβ amyloid burden was reduced in old SDPM1-immunized mice (Fig. 2 and Fig. 3). Swim speeds and general locomotor activity (open field tests) were unchanged for all groups (Fig. 4C and F, Fig. S8). Thus, altered escape time and probe tests were not a function of altered swimming or movement ability.
Figure 4. Immunization with SDPM1 prior to plaque onset improves cognitive performance in Morris water maze tests.
Young (beginning at 5 mo and ending at 12 mo, A–C) or Old (beginning at 12mo and ending at 18 mo, D–F) Mock-, SDPM1-, and SDPM2-immunized APPswePSEN1(A246E) (APP/PS1) mice were compared to non-immunized wild type (WT) animals in the Morris water maze test for escape latency (A. D), probe test (B, E) and swim speed (C, F). Errors are SEM for n=7–12 animals per condition with n=4 measurements per animal per condition.
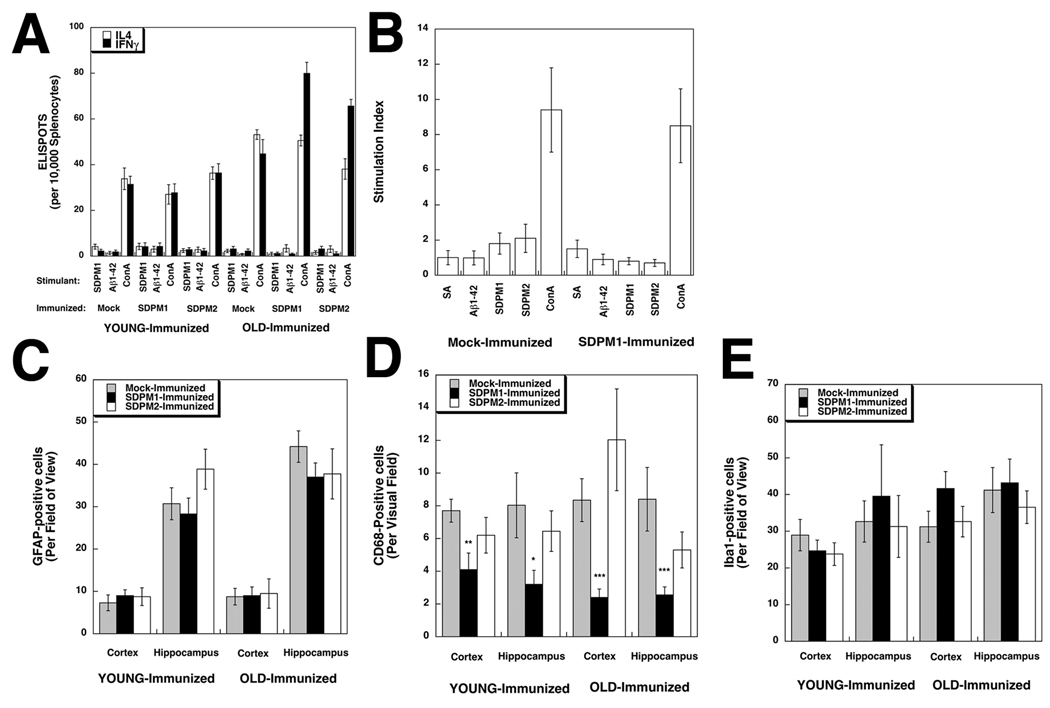
Immunization with SDPM1 does not elicit T cell activation to SDPM1 or Aβ1–42 peptide or stimulate brain inflammation
We next determined if use of SDPM1 as an immunogen would stimulate unwanted T cell responses, as has occurred in some AD patients immunized with AN1792(Orgogozo et al., 2003). We assessed T cell responses in three ways. First, splenocytes were isolated from SDPM1-, SDPM2- and mock-immunized APPswePSEN1(A246E) animals (both for young animals and old animals with adjuvant) and T cell responses to SDPM1 or Aβ1–42 peptide were assessed by ELISPOT assay for activation of Interleukin 4 (IL4) or Interferon gamma (IFNγ) expression. IL4 and IFNγ responses were negligible to SDPM1 and Aβ1–42 and were no different than responses with no peptide added (Fig. 5A and S9). Addition of Concanavalin A (ConA) was used as a positive control, and this induced a one-log increase in signal for either IL4 or IFNγ. Second, we compared splenocytes from young SDPM1-immunized APPswePSEN1(A246E) animals (with adjuvant) and mock-immunized controls for T cell proliferation using a 3H thymidine cell division assay (Fig. 5B). Addition of Aβ1-42, SDPM1, or SDPM2 stimulated no significant increase in 3H thymidine uptake. Here again, ConA served as a positive control, yielding a stimulation index almost ten-fold above background. Last, we immunostained brains (young and old, immunized with adjuvant) using antibodies to GFAP (to mark astrocytes), CD68 (to mark activated microglia) and Iba1 (to mark all microglia) (Fig. 5C–E and Fig. S10–16). Quantification of GFAP (Fig. 5C) and Iba1 (Fig. 5E) staining signal showed no significant changes between mock-, SDPM1- or SDPM2-immunized animals, young or old, while immunostaining for CD68 was significantly reduced (by 50% or more) in SDPM1-immunized cortex and hippocampus (both young and old) (Fig. 5D). The reduced staining for CD68 suggests that activated microglia were less prevalent in SDPM1-immunized brain, consistent with the fact that there was reduced amyloid plaque burden (Fig. 2D and 2E). We found no evidence for brain parenchymal staining using antibodies to CD3, CD4, CD8, or B220, suggesting no T or B cell brain infiltrates were present (not shown). These data, in aggregate, suggest that immunization with SDPM1 does not lead to increased T cell or inflammatory responses in APPswePSEN1(A246E) brain.
Figure 5. Immunization with SDPM1 does not stimulate T cell responses to SDPM1 or Aβ1–42 or induce brain inflammation in APPswePSEN1(A246E) transgenic mice.
(A) Quantification of ELISPOT assays for Interleukin 4 (IL4)-positive and Interferon gamma (IFNγ)-positive T cells after stimulation with SDPM1, Aβ1–42 peptide, or Concanavalin A (ConA), subtracted from no peptide control. (B) Splenocytes were stimulated with streptavidin (SA), Aβ1–42, SDPM1, SPDM2, or Concanavalin A (ConA), after which cells were incubated with 3H thymidine. 3H incorporation was assayed to determine stimulation of cell division of T lymphocytes, relative to serum-containing media alone. Cells staining positive for GFAP (C), CD68 (D), or Iba1 (E) were quantified. Errors are SEM for n=7–12 animals per condition for n=3 measurements per animal in (A), 4 measurements per animal in (B), and 10 measurements per animal in C–E.
Discussion
While immunization with Aβ amyloid peptide has been shown to lower amyloid plaque burden in AD patients(Hock et al., 2003; Nicoll et al., 2006) and in AD animal models(Bard et al., 2003; Schenk et al., 1999), the use of Aβ peptides as immunogens is complicated by the fact that Aβ amyloid is a spontaneously aggregating complex mixture of quaternary protein forms. This can have three unwanted consequences. First, immunization with such a complex mixture may stimulate immune responses to Aβ species that may be unproductive in lowering amyloid plaque burden or induce unwanted microglial or lymphocyte activation and brain inflammation. Use of a complex mixture of Aβ protein species as an immunogen may also lower immunogenicity for the therapeutically most relevant Aβ amyloid forms merely by dilution. Second, Aβ immunization risks potential autoimmune responses against Aβ peptide present in endogenous APP protein, which is ubiquitously expressed and, along with APLP1 and APLP2, essential for life(Heber et al., 2000). Last, the expression of APP in many cell types, along with BACE and presenilins, leads to the production of low endogenous levels of Aβ amyloid that may induce tolerance to Aβ amyloid as an immunogen, making immunization with Aβ peptides unproductive. The AN1792 clinical trial showed a majority of patients, about 80%, did not respond by developing increased Aβ antibody titers despite the use of adjuvant therapy(Gilman et al., 2005), suggesting immunogen potency or tolerance were significant issues that may have resulted from the complexities of Aβ amyloid protein structure.
Here we describe the therapeutic efficacy of a new AD vaccine strategy utilizing SDPM1, an Aβ amyloid-binding and blocking peptide, to induce therapeutic peptide mimotope antibodies. Immunization with SDPM1 can induce monoclonal antibodies such as P4D6 that have the same biological effects as the immunogenic peptide; Both SDPM1 and P4D6 bind low molecular weight Aβ amyloid, including Aβ tetramers, and block subsequent Aβ amyloid aggregation. This suggests that there is a binding interface on small aggregates of Aβ amyloid that is reflected by the SDPM1 peptide and P4D6 in a manner similar to an antibody/anti-ideotype response as has been described in other systems(Ponomarenko et al., 2007). Jerne first suggested that every antibody has an anti-ideotype antibody directed to its same parotype(Jerne, 1974). In an analogous manner, peptides may be used elicit antibodies that are mimotopes of the original peptide binding site(Riemer and Jensen-Jarolim, 2007). P4D6 has all of the properties to be a mimotope of SDPM1. The identification of such mimotope antibodies shows that SDPM1 can be used as an immunogen to produce antibodies that both bind small Aβ amyloid aggregates and prevent their subsequent maturation into amyloid plaques. Because SDPM1 is an engineered peptide with no known matches above 50% in known protein databases, it could be more immunogenic than Aβ amyloid in inducing specific types of anti-Aβ antibodies. Alternatively, both SDPM1 and P4D6 may be exact mimics of a particular quaternary Aβ amyloid structure. If so, immunization with SDPM1 would be no different than immunizing with other Aβ amyloid peptides or antibodies. Even so, SDPM1 would still provide certain advantages as an immunogen. For example, SDPM1 does not make larger oligomeric structures in Thioflavin T assays, as Aβ1–40/42 amyloid does. Further, all animals immunized with SDPM1 developed some level of SDPM1 -specific titer, while no animal showed an endogenous SDPM1 -specific titer prior to immunization. Last, as SDPM1 recognizes an interface present on small aggregates of Aβ amyloid, it likely is more specific in the types of antibodies it induces than Aβ1–40/1–42 peptide mixtures, which can spontaneously form multiple quaternary species. P4D6 preferentially binds Aβ1–40 and Aβ1–42 amyloid tetramers relative to high MW amyloid and monomeric Aβ1–40/42 peptide. While one monoclonal antibody does not reflect the entirety of the SDPM1 immune response, immunization with SDPM1 clearly can stimulate the production of anti-Aβ amyloid antibodies that can block Aβ aggregation. Whether such antibodies are directly responsible for lowering plaque burden will require further testing using passive immunotherapy approaches.
The fact that we see improvement in cognitive learning and memory tests when APPswePSEN1(A246E) mice are vaccinated with SDPM1 prior to amyloid plaque formation, but see no improvement when mice are immunized after amyloid plaque burden is significant, suggests that SDPM1 would only be useful as a preventative vaccine to inhibit disease progression, as opposed to a treatment that would reverse disease once present. SDPM1 vaccination of old animals with significant plaque burden did effectively lower Aβ protein and amyloid plaque burden throughout the brain. This further suggests that the damage resulting from Aβ amyloid already present in these older animals irreversibly changed their cognitive abilities. Alternatively, the lowered cognitive abilities in these mice simply may simply be unaffected by SDPM1 vaccination. Regardless, these results suggest that SDPM1 likely would only work in AD patients prior to onset of full disease. The improvements in Water Maze measures, both with regard to reduced latency to reach platform, a measure of learning, and improved time in platform quadrant during a probe test, a measure of memory of the learned skill, rivaled the cognitive improvements seen using Aβ immunotherapy or active Aβ peptide vaccination in APP or APP/PSEN1 transgenic mouse lines(Lee et al., 2006; Lemere, 2009; Morgan et al., 2000; Mouri et al., 2007; Savonenko et al., 2005; Sigurdsson et al., 2004; Wilcock et al., 2009).
It is very important to point out that while APPswePSEN1(A246E) mice are an excellent model for development of increased amyloid plaque burden and a relatively good model for altered cognitive and synaptic function, these mice do not develop two very important aspects of Alzheimer’s disease pathology – the formation of neurofibrillary tangles and neurodegeneration(Morrissette et al., 2009). Thus, our results must be viewed within the context of the limitations of the animal model we have used. The formation of neurofibrillary tangles and neuronal cell death may nevertheless be present in AD as a result of Aβ1–40/1–42 peptide production. For example, Aβ immunotherapy can remove neurofibrillary tangles from APPswePSEN1(M146V)MAPT(P301L) triple transgenic mice(Oddo et al., 2004)) and reduction of Tau in APP transgenic mice can prevent cognitive decline(Roberson et al., 2007). The presence of a linear pathway from Aβ amyloid overproduction to neurofibrillary tangle formation, however, is far from proved. To the extent that this is the case, it is likely that SDPM1 could prevent these features of AD as well, as its ability to inhibit Aβ1–40/1–42 peptide and plaque burden would similarly inhibit their down-stream effects. To the extent that neurofibrillary tangles and neurodegeneration are generated by Aβ-independent mechanisms in AD, it is likely that SDPM1 would be ineffective in altering disease course, as these aspects are the most likely ones to contribute to the development of dementia. It will be useful to test this approach in newer triple transgenic models that contain mutant human Tau transgenes, as these mice possess neurofibrillary tangles that are similar to those found in AD brain(Jaworski et al.).
The lack of T cell responses to the immunogenic SDPM1 peptide and to the Aβ1–42 peptide, coupled with the lack increased inflammatory cells in the brains of SDPM1-immunized mice, suggests that vaccination with SDPM1 does not stimulate the immunological side effects that caused the cessation of the AN-1792 clinical trial(Orgogozo et al., 2003). The development of meningoencephalitis in 6% of patients treated with AN-1792 strongly suggested the induction of a T helper 1 (Th1) immune response; QS21, the adjuvant used in that trial, strongly activates Th1 T cell responses, and Th1 responses can activate encephalitogenic T cells and induce inflammation in the central nervous system. In our study, almost all SDPM1-specific antibodies induced in young or old APPswePSEN1(A246A) transgenic mice were of the IgG1 subtype; The 10:1 ratio of IgG1 to IgG2a in these experiments is reflective of a Th2 type response, as Th1 responses typically induce IgG2a antibodies while Th2 responses typically induce IgG1 (or IgG2b)(Finkelman et al., 1990). Because the T cell epitopes in Aβ1–40/1–42 are present in the C-terminal half of the peptide and are absent in the N-terminal 15 amino acids(Monsonego et al., 2001; Monsonego et al., 2003), a number of strategies aimed at using only the N-terminal half of Aβ peptide are currently being investigated to minimize Th1 T cell responses. Immunization with Aβ1–5 multimers (conjugated with ovalbumin T cell epitope (OVAT)(Bard et al., 2003) or with murine leukemia virus particles and PDGF protein(Bach et al., 2009)), with Aβ1–15 multimers (conjugated with pan HLA DR-binding peptide (PADRE) and Alum(Agadjanyan et al., 2005), delivered in liposomes(Muhs et al., 2007), or delivered intranasally(Maier et al., 2006)), or with Aβ1–28 multimers (conjugated to Mannan(Ghochikyan et al., 2006b)) all produced minimal Th1 responses while reducing Aβ plaque burden. Development of AFFITOPE vaccines using six amino acid Aβ-like peptides showed similarly positive effects(Schneeberger et al., 2009). Immunization of Aβ1–42 delivered using techniques that bias towards Th2 responses (intranasal immunization(Weiner et al., 2000), transcutaneous immunization with cholera toxin(Nikolic et al., 2007), or DNA vaccination(Qu et al., 2007)) also show promise at avoiding unwanted Th1 T cell activation while reducing Aβ amyloid burden. Thus, while SDPM1 is truly unique in that it bears no similarity to any portion of the Aβ1–40 or Aβ1–42 peptide, many other approaches using shortened versions of the Aβ peptide may also prove useful in ultimately avoiding unwanted immune responses.
Supplementary Material
Acknowledgements
This work is supported by grants from the National Institutes of Health (NIAMS;AR050202 and AR049722 to PTM). We would like to thank to Christopher Walker (Center for Vaccines and Immunology, Nationwide Children’s Hospital) for assistance and advice with immune cell assays and Christopher Phiel (Center for Cell and Developmental Biology, Nationwide Children’s Hospital) for APP expression vectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agadjanyan MG, et al. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- Bach P, et al. Vaccination with Abeta-displaying virus-like particles reduces soluble and insoluble cerebral Abeta and lowers plaque burden in APP transgenic mice. J Immunol. 2009;182:7613–7624. doi: 10.4049/jimmunol.0803366. [DOI] [PubMed] [Google Scholar]
- Bachman DL, et al. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42:115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- Bard F, et al. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghorn S, et al. Globular amyloid beta-peptide oligomer -a homogenous and stable neuropathological protein in Alzheimer's disease. J Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- Buttini M, et al. Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer's disease. J Neurosci. 2005;25:9096–9101. doi: 10.1523/JNEUROSCI.1697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Deshpande A, et al. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, et al. Reduced levels of amyloid beta-peptide antibody in Alzheimer disease. Neurology. 2001;57:801–805. doi: 10.1212/wnl.57.5.801. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Games D, et al. Mice as models: transgenic approaches and Alzheimer's disease. J Alzheimers Dis. 2006;9:133–149. doi: 10.3233/jad-2006-9s316. [DOI] [PubMed] [Google Scholar]
- Geylis V, et al. Human monoclonal antibodies against amyloid-beta from healthy adults. Neurobiol Aging. 2005;26:597–606. doi: 10.1016/j.neurobiolaging.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Ghochikyan A, et al. Prototype Alzheimer's disease epitope vaccine induced strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant switch. Vaccine. 2006a;24:2275–2282. doi: 10.1016/j.vaccine.2005.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghochikyan A, et al. Abeta-immunotherapy for Alzheimer's disease using mannan-amyloid-Beta peptide immunoconjugates. DNA Cell Biol. 2006b;25:571–580. doi: 10.1089/dna.2006.25.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Gong Y, et al. Alzheimer's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci U S A. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, et al. Oxidation of Abeta and plaque biogenesis in Alzheimer's disease and Down syndrome. Neurobiol Dis. 2001;8:792–806. doi: 10.1006/nbdi.2001.0431. [DOI] [PubMed] [Google Scholar]
- Heber S, et al. Mice with combined gene knock-outs reveal essential and partially redundant functions of amyloid precursor protein family members. J Neurosci. 2000;20:7951–7963. doi: 10.1523/JNEUROSCI.20-21-07951.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Hyman BT, et al. Autoantibodies to amyloid-beta and Alzheimer's disease. Ann Neurol. 2001;49:808–810. doi: 10.1002/ana.1061. [DOI] [PubMed] [Google Scholar]
- Jaworski T, et al. Alzheimer's disease: Old problem, new views from transgenic and viral models. Biochim Biophys Acta. doi: 10.1016/j.bbadis.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Jerne NK. Towards a network theory of the immune system. Ann Immunol (Paris) 1974;125C:373–389. [PubMed] [Google Scholar]
- Kang CK, et al. Identification of peptides that specifically bind Abeta1–40 amyloid in vitro and amyloid plaques in Alzheimer's disease brain using phage display. Neurobiol Dis. 2003;14:146–156. doi: 10.1016/s0969-9961(03)00105-0. [DOI] [PubMed] [Google Scholar]
- Kutzler MA, et al. Mapping of immune responses following wild-type and mutant ABeta42 plasmid or peptide vaccination in different mouse haplotypes and HLA Class II transgenic mice. Vaccine. 2006;24:4630–4639. doi: 10.1016/j.vaccine.2005.08.036. [DOI] [PubMed] [Google Scholar]
- Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, et al. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- Lemere CA. Developing novel immunogens for a safe and effective Alzheimer's disease vaccine. Prog Brain Res. 2009;175:83–93. doi: 10.1016/S0079-6123(09)17506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, et al. Novel Abeta immunogens: is shorter better? Curr Alzheimer Res. 2007;4:427–436. doi: 10.2174/156720507781788800. [DOI] [PubMed] [Google Scholar]
- Maier M, et al. Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer's disease mouse model in the absence of an Abeta-specific cellular immune response. J Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PT, et al. Overexpression of Galgt2 in skeletal muscle prevents injury resulting from eccentric contractions in both mdx and wild-type mice. Am J Physiol Cell Physiol. 2009;296:C476–C488. doi: 10.1152/ajpcell.00456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego A, et al. Immune hyporesponsiveness to amyloid beta-peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:10273–10278. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego A, et al. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J Clin Invest. 2003;112:415–422. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto N, et al. Conformation-sensitive antibodies against alzheimer amyloid-beta by immunization with a thioredoxin-constrained B-cell epitope peptide. J Biol Chem. 2007;282:11436–11445. doi: 10.1074/jbc.M609690200. [DOI] [PubMed] [Google Scholar]
- Morgan D. Immunotherapy for Alzheimer's disease. J Alzheimers Dis. 2006;9:425–432. doi: 10.3233/jad-2006-9s348. [DOI] [PubMed] [Google Scholar]
- Morgan D, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Morrissette DA, et al. Relevance of transgenic mouse models to human Alzheimer disease. J Biol Chem. 2009;284:6033–6037. doi: 10.1074/jbc.R800030200. [DOI] [PubMed] [Google Scholar]
- Mouri A, et al. Oral vaccination with a viral vector containing Abeta cDNA attenuates age-related Abeta accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. FASEB J. 2007;21:2135–2148. doi: 10.1096/fj.06-7685com. [DOI] [PubMed] [Google Scholar]
- Muhs A, et al. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc Natl Acad Sci U S A. 2007;104:9810–9815. doi: 10.1073/pnas.0703137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki H, et al. Concentration-dependent inhibitory effects of apolipoprotein E on Alzheimer's beta-amyloid fibril formation in vitro. Biochemistry. 1997;36:6243–6250. doi: 10.1021/bi9624705. [DOI] [PubMed] [Google Scholar]
- Nath A, et al. Autoantibodies to amyloid beta-peptide (Abeta) are increased in Alzheimer's disease patients and Abeta antibodies can enhance Abeta neurotoxicity: implications for disease pathogenesis and vaccine development. Neuromolecular Med. 2003;3:29–39. doi: 10.1385/nmm:3:1:29. [DOI] [PubMed] [Google Scholar]
- Nguyen HH, et al. Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice. Proc Natl Acad Sci U S A. 2002;99:5616–5621. doi: 10.1073/pnas.082613599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, et al. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- Nikolic WV, et al. Transcutaneous beta-amyloid immunization reduces cerebral beta-amyloid deposits without T cell infiltration and microhemorrhage. Proc Natl Acad Sci U S A. 2007;104:2507–2512. doi: 10.1073/pnas.0609377104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi A, et al. Isolation and characterization of patient-derived, toxic, high-mass amyloid {beta}-protein (A{beta}) assembly from Alzheimer's disease brains. J Biol Chem. 2009 doi: 10.1074/jbc.M109.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, et al. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Pan W, et al. Antibodies to beta-amyloid decrease the blood-to-brain transfer of beta-amyloid peptide. Exp Biol Med (Maywood) 2002;227:609–615. doi: 10.1177/153537020222700808. [DOI] [PubMed] [Google Scholar]
- Ponomarenko NA, et al. Anti-idiotypic antibody mimics proteolytic function of parent antigen. Biochemistry. 2007;46:14598–14609. doi: 10.1021/bi7013954. [DOI] [PubMed] [Google Scholar]
- Qu BX, et al. Abeta42 gene vaccine prevents Abeta42 deposition in brain of double transgenic mice. J Neurol Sci. 2007;260:204–213. doi: 10.1016/j.jns.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemer AB, Jensen-Jarolim E. Mimotope vaccines: epitope mimics induce anti-cancer antibodies. Immunol Lett. 2007;113:1–5. doi: 10.1016/j.imlet.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Savonenko A, et al. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18:602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Schenk D, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schenk DB, et al. A beta immunotherapy: Lessons learned for potential treatment of Alzheimer's disease. Neurodegener Dis. 2005;2:255–260. doi: 10.1159/000090365. [DOI] [PubMed] [Google Scholar]
- Schneeberger A, et al. Development of AFFITOPE vaccines for Alzheimer's disease (AD)--from concept to clinical testing. J Nutr Health Aging. 2009;13:264–267. doi: 10.1007/s12603-009-0070-5. [DOI] [PubMed] [Google Scholar]
- Seabrook TJ, et al. Dendrimeric Abeta1–15 is an effective immunogen in wildtype and APP-tg mice. Neurobiol Aging. 2007;28:813–823. doi: 10.1016/j.neurobiolaging.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, et al. An attenuated immune response is sufficient to enhance cognition in an Alzheimer's disease mouse model immunized with amyloid-beta derivatives. J Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaton M, Tamagno E. The molecular link between beta- and gamma-secretase activity on the amyloid beta precursor protein. Cell Mol Life Sci. 2007;64:2211–2218. doi: 10.1007/s00018-007-7219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD. The pathogenesis of Alzheimer disease: an alternative to the amyloid hypothesis. J Neuropathol Exp Neurol. 1996;55:1023–1025. [PubMed] [Google Scholar]
- Tucker SM, et al. Limited clearance of pre-existing amyloid plaques after intracerebral injection of Abeta antibodies in two mouse models of Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:30–40. doi: 10.1097/nen.0b013e31815f38d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL, et al. Nasal administration of amyloid-beta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer's disease. Ann Neurol. 2000;48:567–579. [PubMed] [Google Scholar]
- Weksler ME, et al. Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp Gerontol. 2002;37:943–948. doi: 10.1016/s0531-5565(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, et al. Amyloid reduction by amyloid-beta vaccination also reduces mouse tau pathology and protects from neuron loss in two mouse models of Alzheimer's disease. J Neurosci. 2009;29:7957–7965. doi: 10.1523/JNEUROSCI.1339-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, et al. Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta. J Neurosci. 2009;29:11393–11398. doi: 10.1523/JNEUROSCI.2021-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, et al. The synaptic CT carbohydrate modulates binding and expression of extracellular matrix proteins in skeletal muscle: Partial dependence on utrophin. Mol Cell Neurosci. 2009 doi: 10.1016/j.mcn.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.