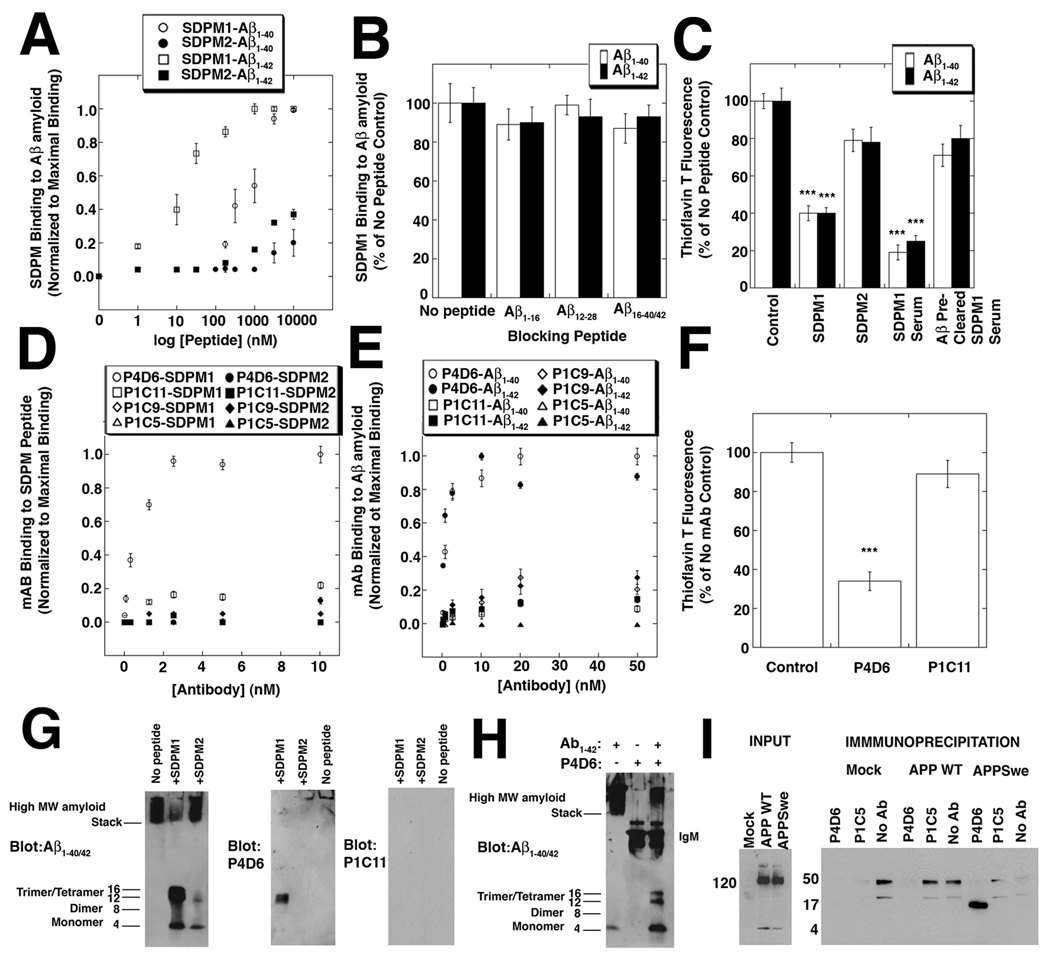
Figure 1. SDPM1, and SDPM1-mimotope antibodies bind low molecular weight Aβ1–40/1–42 amyloid and block subsequent Aβ amyloid aggregation.
(A) Binding of the SDPM1 and the SDPM2 peptide to Aβ1–40 and Aβ1–42 amyloid. (B) Addition of molar excess of monomeric Aβ1–16, Aβ12–28, Aβ16–40, or Aβ16–42 peptide does not block SDPM1 binding to Aβ1–40 or Aβ1–42 amyloid. (C) Addition of SDPM1 or SDPM1 immune serum with monomeric Aβ1–40 or Aβ1–42 amyloid blocks aggregation of Aβ amyloid. Pre-clearing of SDPM1-immune serum on Aβ1–42 amyloid removes its blocking ability. (D) P4D6 binds SDPM1 (open circles) but not SDPM2 (closed circles), while P1C11, P1C9, and P1C5 do not bind either SDPM1 or SDPM2 with high affinity. (E) P4D6 shows high affinity binding to Aβ1–40 (open circles) and Aβ1–42 (closed circles) amyloid, while P1C11, P1C9, and P1C5 do not. (F) P4D6 blocks amyloid aggregation of Aβ1–42 peptide in aqueous solution, while P1C11 does not. (G) Addition of SDPM1 to Aβ1–42 peptide blocks formation of high molecular weight (MW) amyloid formation and traps 12 kDa (trimer) and 16 kDa (tetramer) forms. These forms are blotted by P4D6 but not by P1C11. (H) Addition of P4D6 with Aβ1–42 peptide blocks high MW amyloid formation and traps trimer/tetramer Aβ amyoid forms. (I) COS7 cells were either mock-transfected or transfected with a V5-tagged cDNA for amyloid precursor protein (APP WT) or the Swedish early onset AD mutant (APPswe). Input from whole cell lysates, blotted with a V5 antibody, showed equivalent levels of APP WT and APPswe were present. Equivalent amounts of non-ionic detergent cell lysates were precipitated with resin alone (No Ab), P4D6, or P1C5, then blotted with antibody to Aβ1–42. Only P4D6 precipitated low molecular weight (16kDa) Aβ amyoid. Errors are SEM for n=4 (A, B), 6 (D–E), or 3 (C, F) measurements per condition.