Fig. 1.
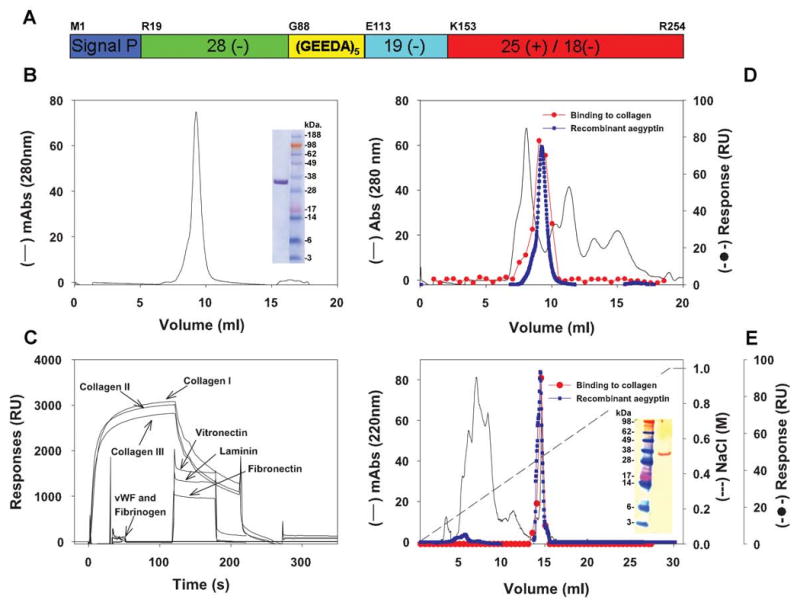
Molecular characterization of aegyptin and identification in the Ae. aegypti salivary gland. (A) Schematic representation of aegyptin with typical acidic N-terminus, (GEEDA) repeats, and basic carboxyterminal depicted. (B) Purification of recombinant aegyptin after Ni2+ agarose and gel-filtration columns. Inset: NU-PAGE of purified aegyptin under denaturating conditions. (C) Plasmon resonance experiments: aegyptin interacts with collagen (I-III) but not with vitronectin, laminin, fibronectin, vWf, and fibrinogen. (D) Gel filtration of salivary gland homogenate (—) and identification of the active fraction that binds to collagen (–•–). The elution pattern of recombinant aegyptin is superimposed (–•–). (E) Anion-exchange chromatography of the active fraction obtained in (D) and identification of collagen-binding activity (–•–). The elution pattern of recombinant aegyptin is superimposed (–•–). Inset: Western blot for detection of aegyptin in the salivary gland using polyclonal antiaegyptin antibodies.
