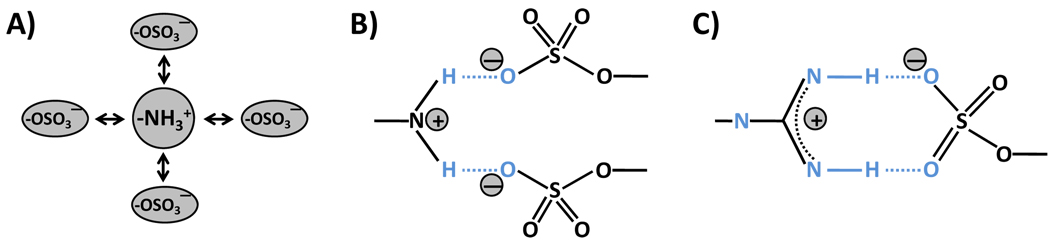
Figure 2. Interaction of sulfate group(s) with positively charged nitrogen containing groups.
A) The interaction relies solely on the ionic charges on the two groups. This interaction is defined as Coulomb-type interaction and occurs over a longer range in comparison to other atomic interactions. It is isotropic and does not involve any geometrical constraints. B) The interaction between a Lys or an Arg with one or more sulfate groups may sandwich an H atom resulting in the formation of a hydrogen bond. This H-bond may not be linear, yet provides sufficient energy to engineer specificity of recognition. The stoichiometry of interaction here may be 1:1 or 1:2 per nitrogen atom. C) For arginine, a linear H-bond geometry is feasible generating significant bond energy and greater specificity of recognition. The stoichiometry of interaction here is 1:1. Geometry C) is expected to be most stable.