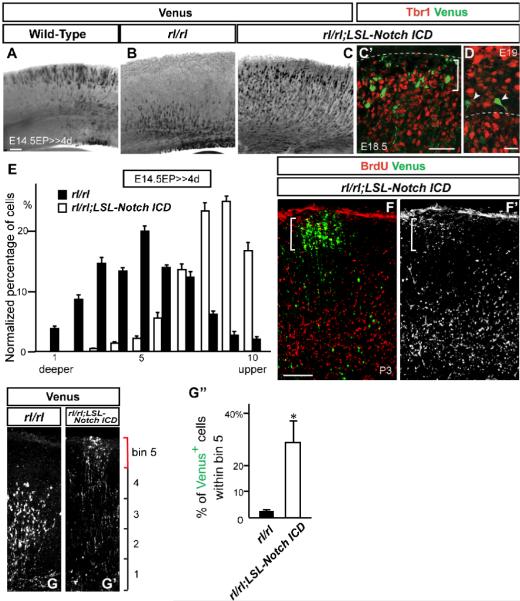
Figure 5. Replenishing of Notch ICD mitigates neuronal migration defects in Reeler.
(A-D) Immunostaining for Venus (black, green) and Tbr1 (red) in cortical slices of indicated genotypes 4 days (A-C’) or 4.5 days (D) post-electroporation (with pTα1-Cre-IRES-Venus). (D) Higher magnification view around the SPP. Dashed lines in (C’) and (D) indicate the pial surface and the border between the SPP and CP, respectively. Bracket in (C’) shows the SPP region. Note that electroporated Venus+ cells did not change their fate to Tbr1+ early-born neurons (C’, D). (E) Quantification of neuronal distribution showed significantly more cells in upper CP of rl/rl;LSL-Notch ICD compared to rl/rl cortex (K-S test, p<0.0001; Repeated Measures ANOVA, F(9,54)=18.91, p<0.0001). The data represent the mean ± SEM of 6 brains each. (F, F’) Immunostaining for Venus (green) and BrdU (red, white) in P3 cortical slices of rl/rl;LSL-Notch ICD electroporated with pTα1-Cre-IRES-Venus. Note that Venus+/BrdU+ cells (indicated by bracket) located over BrdU+ cells in surrounding lower layers. (G, G’) Venus immunostaining in P3 cortical slices of rl/rl and rl/rl;LSL-Notch ICD electroporated with pTα1-Cre-IRES-Venus. (G”) Quantification of Venus+ neurons located in the upper part of the CP (within the bin 5 indicated by a red bracket in G, G’. Entire thickness of the cortex was subdivided into 5 bins.) The data represent the mean ± SEM of 3 brains each. *p<0.05, Student’s t-test. Bars (μm) = 100 (A-C’, F-G’), 20 (D).