Fig. 2.
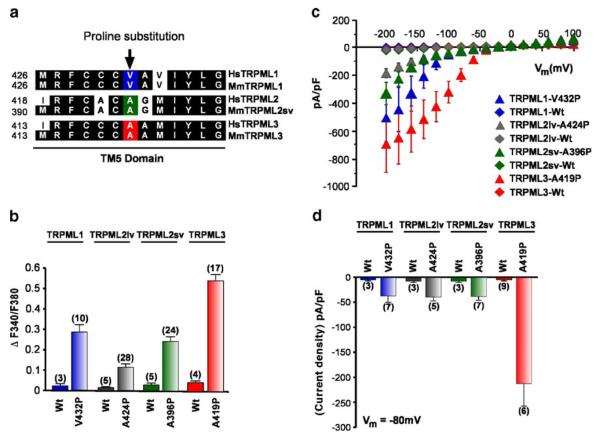
Constitutive channel activity of TRPML varitint-waddler (Va) mutant proteins. a Sequence alignment (clustal W) of the inner half of the TM5 domain of the three TRPML proteins. Conserved amino-acid residues are highlighted in black. Arrow indicates the position of the proline substitution from the varitint-waddler mutation A419P in TRPML3 and equivalent positions for TRPML1 and TRPML2 proteins. b Ca2+-imaging experiments showing intracellular Ca2+ levels of HEK-293 cells expressing either wild-type TRPML1, TRPML2lv (long variant), TRPML2sv (short variant), or TRPML3, and TRPML-Va mutant versions TRPML1-V432P, TRPML2lvA424P, TRPML2sv-A396P, and TRPML3-A419P. All experiments were performed 15-20 h after transfection due to cytotoxic effect of the mutation. Data are represented as means ± SEM, N=number in parenthesis. c Steady-state current-voltage plots of constitutively active whole-cell currents elicited by TRPML1-V432P, TRPML2svA396P, TRPML2lv-A424P, and TRPML3-A419P mutant proteins compared with their respective wild-type versions in response to 10 ms voltage steps from a holding potential of +10 mV between −200 mV and +100 mV in 20 mV incremental steps and normalized by cell capacitance (pF). All TRPML-Va mutants showed inward rectification while wild-type TRPMLs did not elicit any response. d Average inward current densities at −80 mV of all TRPML-Va mutant and wild-type proteins shown in panel c and normalized by pF. The TRPML3-Va mutation showed higher current density compared with the other TRPML-Va mutants. Data are represented as means ±SEM, N=numbers in parenthesis. All experimental procedures are outlined in the Materials and methods section