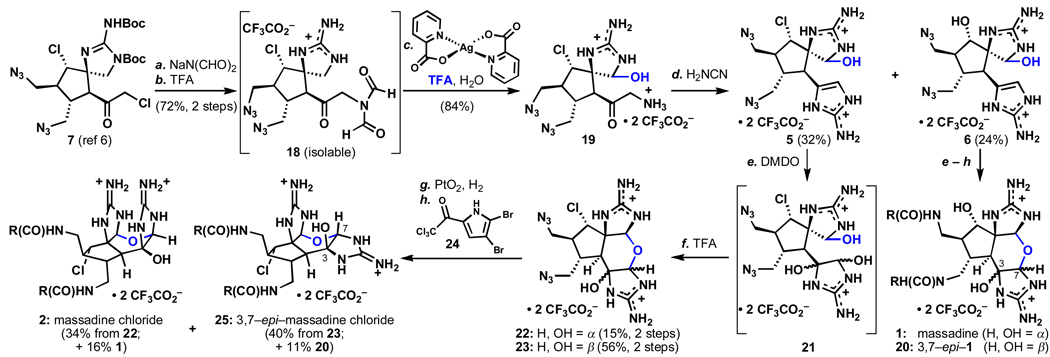
Scheme 1.
Total syntheses of the massadines.a
aReagents and conditions: (a) Sodium diformylamide (1.2 equiv), TBAI (0.1 equiv), THF, 23 °C, 2 h, 72%; (b) 2:1 TFA:DCM, 23 °C, 2 h, quant; (c) silver(II)picolinate (2.5 eq), 9:1 H2O:TFA, 23 °C, 35 min, then TFA (to 1:1 v:v), 38 °C, 18 h, 84%; (d) cyanamide (excess), 0.2 M NaOH (to pH 5.0), 78 °C, 2 h, 24% 5, 32% 6; (e) DMDO (1.3 equiv), 9:1 H2O:TFA, 0 °C, 1h (f) TFA, 23 °C, 3 h, 71% over two steps, 1:3.7 22:23, (65% for −OH series, 1:1.9); (f) PtO2 (0.3 equiv), H2 (1 atm), 19:1 H2O:TFA, 1 h, 23 °C; (g) 24 (16 equiv), iPr2EtN (16 equiv) DMF, 14 h, 23 °C, 34% (22→2), 44% (23→25) (40% for 1 and 40% for 20, −OH series). TBAI = tetrabutylammonium iodide, TFA = trifluoroacetic acid, DMDO = dimethyldioxirane, R = 5–(2,3–dibromopyrrole).