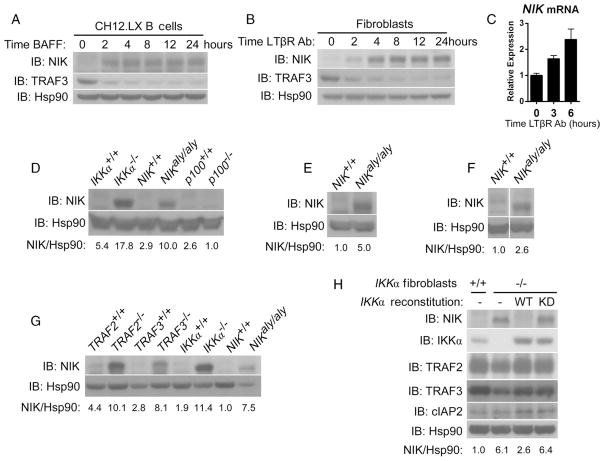
Fig. 1.
IKKα inhibits NIK. (A and B) Long-term receptor ligation leads to the accumulation of a constant amount of NIK, in the absence of the resynthesis of TRAF3, as assayed by Western blotting in CH12.LX B cells treated with hBAFF (100 ng/ml) (A) or in murine fibroblasts treated with an agonistic antibody against LTβR (2 μg/ml) (B). (C) The abundance of NIK mRNA was assayed by quantitative polymerase chain reaction (PCR) assay in murine fibroblasts and did not decrease after treatment with agonistic antibody against LTβR (2 μg/ml). (D) NIK is basally stable in untreated fibroblasts from IKKα−/− and NIKaly/aly but not wild-type or p100−/− fibroblasts as assessed by Western blotting. Values reported for the quantitation of bands here (in numbers beneath the blots) and in all other cases are relative arbitrary units as described in Materials and Methods. (E and F) Enhanced stability of NIK in freshly isolated splenic B cells (E) and primary bone marrow macrophages treated with recombinant mRANK (100 ng/ml) (F) from NIKaly/aly compared to that in cells isolated from wild-type mice. (G) Enhanced basal stability of NIK from IKKα−/− and NIKaly/aly fibroblasts is comparable to that observed in TRAF2−/− and TRAF3−/− fibroblasts as assayed by Western blotting. (H) Enhanced basal stability of NIK observed in IKKα−/− fibroblasts is dependent on the kinase activity of IKKα as evidenced by retroviral reconstitution of IKKα−/− fibroblasts with pBABE-Empty Vector (−), pBABE-IKKα-WT (WT), or pBABE-IKKα-K44A (KD), the kinase-defective mutant of IKKα.