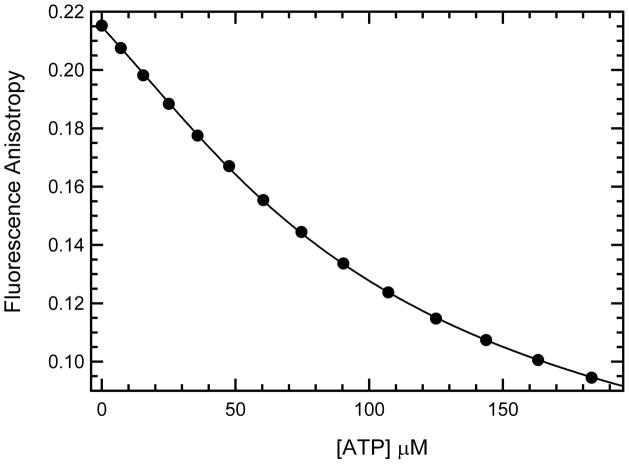
Figure 4.
Binding of ATP to phosphorylated PKR measured by competition fluorescence anisotropy. In this case, ATP was titrated against 39.5 μM of phosphorylated PKR (measured by UV absorption at 280 nm) with 11.3 μM mant-ATP and the fluorescence anisotropy measured with excitation and emission at 360 and 443 nm respectively. Each data point represents and average of 3–4 repeated measurements at the same ATP concentration. The Kd of mant-ATP binding to phosphorylated PKR measured by reverse anisotropy titration and used in the fitting function was 24.6 μM.