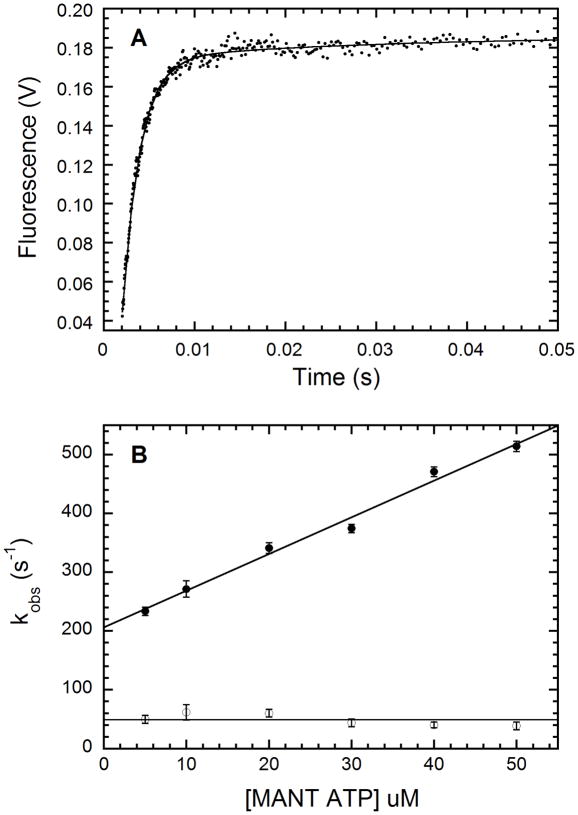
Figure 5.
Kinetics of mant-ATP binding to unphosphorylated wild-type PKR measured by stopped-flow fluorescence spectroscopy. A: Time-dependent changes in fluorescence of 50 μM mant-ATP in the presence of 1 μM unphosphorylated PKR. The mixture was excited at 295 nm and fluorescence emission above 400 nm measured using a cutoff filter. The plot represents average of 12 runs with each run made of 1000 data points taken over 10 s in a log time interval. The solid line is the two exponential fits to the data. The first phase contributed 96% to the total amplitude with the observed rate constant (kobs,1) of 514 ± 9 s−1 while the second phase contributed 4% to the total amplitude with kobs,2 of 39 ± 9 s−1. B: The effect of mant-ATP concentration on kobs. The solid line is a linear fit to the data from two experiments. kobs,1 (●) increases linearly as the concentration of mant-ATP and a least squares fit yields kobs,1:slope = 6.25 ± 0.35 μM−1·s−1 and kobs,1:intercept = 206 ± 11 s−1·kobs,2 (□) is essentially independent of mant-ATP concentration with an average value of 48.9 ± 9.9 s−1.