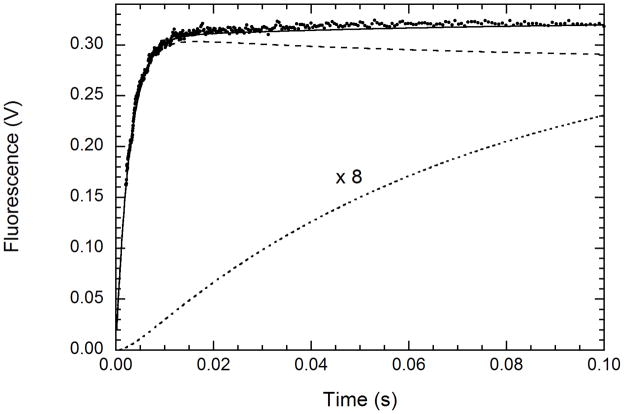
Figure 7.
Simulation of the kinetics of mant-ATP binding to unphosphorylated PKR: [EL] (– –), [E′L] × 8 (.....), [EL] + [E′L] (—). The kinetics were simulated based on a global fit of the experimental data to scheme 1 over the range of 5 – 30 μM mant-ATP, assuming that that EL and E′L contribute equally. The adjustable parameters are the four rate constants and an overall scaling factor, giving best fit values of k1=7.33 ± 0.07 μM−1s−1, k−1 = 137± 2 s−1, k2 =1.8± 0.7 s−1 and k−2 =13.0 ± 10.7 s−1.