Abstract
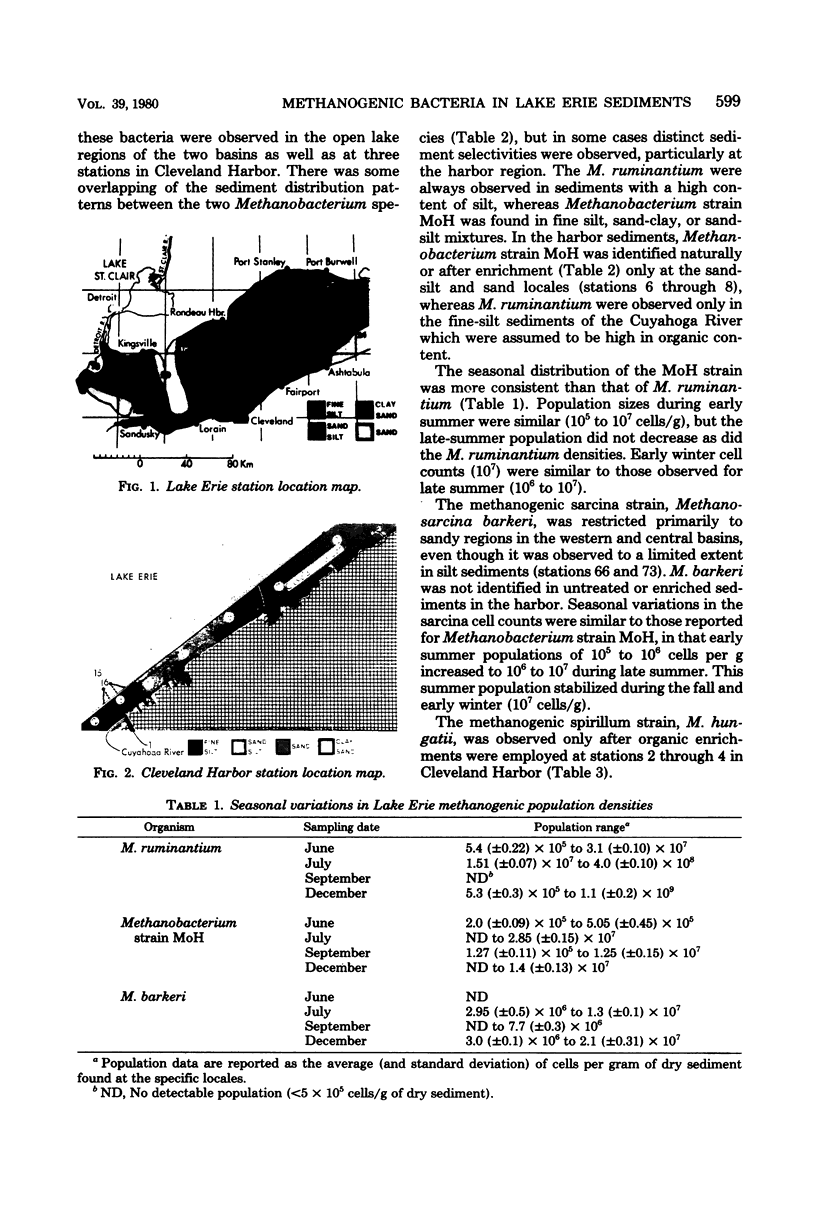
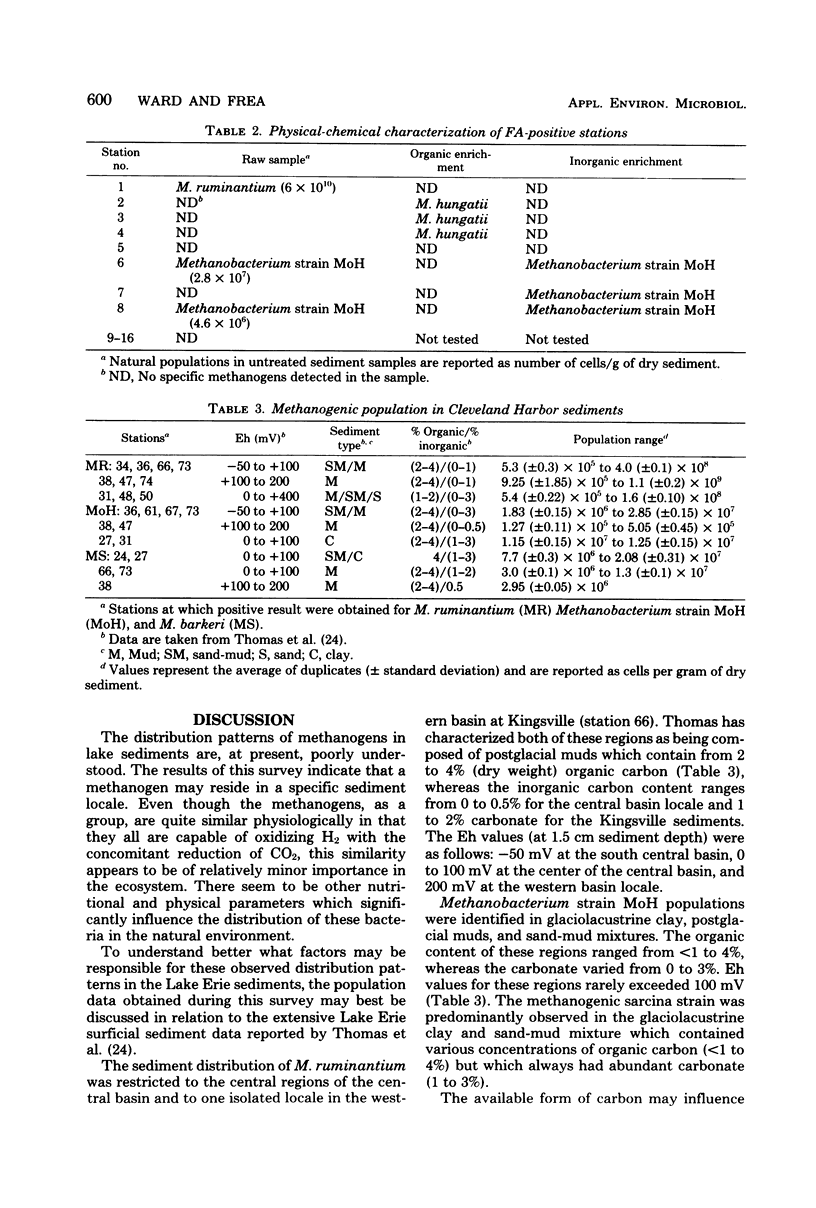
The direct fluorescent-antibody technique was employed to determine the distribution patterns of four species of methanogens in the sediments of Lake Erie and Cleveland Harbor. Methanobacterium ruminantium was the most numerous methanogen found in regions of high-organic-silt sediments. The population of this species ranged from 106 to 109 cells/g of dry sediment. Methanobacterium strain MoH and Methanosarcina barkeri were identified in sand-silt, clay, or sand sediments. These methanogens ranged in density from 106 to 107 cells/g of dry sediment. Methanospirillum hungatii was observed only after an organic enrichment was performed on Cleveland Harbor sediments. The seasonal and selective sediment distribution of these methanogens appears to be related to the type and concentration of carbon as substrate as well as to the activities of heterotrophic and sulfate-reducing bacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant M. P., Campbell L. L., Reddy C. A., Crabill M. R. Growth of desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol. 1977 May;33(5):1162–1169. doi: 10.1128/aem.33.5.1162-1169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. I. Field observations. Antonie Van Leeuwenhoek. 1974;40(2):285–295. doi: 10.1007/BF00394387. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. II. Inhibition experiments. Antonie Van Leeuwenhoek. 1974;40(2):297–306. doi: 10.1007/BF00394388. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E., Prins R. A. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. 3. Experiments with 14C-labeled substrates. Antonie Van Leeuwenhoek. 1974;40(3):457–469. doi: 10.1007/BF00399358. [DOI] [PubMed] [Google Scholar]
- LEWIS V. J., JONES W. L., BROOKS J. B., CHERRY W. B. TECHNICAL CONSIDERATIONS IN THE PREPARATION OF FLUORESCENT-ANTIBODY CONJUGATES. Appl Microbiol. 1964 Jul;12:343–348. doi: 10.1128/am.12.4.343-348.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYLROIE R. L., HUNGATE R. E. Experiments on the methane bacteria in sludge. Can J Microbiol. 1954 Aug;1(1):55–64. doi: 10.1139/m55-008. [DOI] [PubMed] [Google Scholar]
- Mink R. W., Dugan P. R. Tentative identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 Mar;33(3):713–717. doi: 10.1128/aem.33.3.713-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. H., HUNGATE R. E. Isolation and characterization of Methanobacterium ruminantium n. sp. J Bacteriol. 1958 Jun;75(6):713–718. doi: 10.1128/jb.75.6.713-718.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN T. C., BARKER H. A. Studies on the methane fermentation. IX. The origin of methane in the acetate and methanol fermentations by methanosarcina. J Bacteriol. 1951 Jan;61(1):81–86. doi: 10.1128/jb.61.1.81-86.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Bakole R. O., Bohlool B. B. Fluorescent-antibody approach to study of rhizobia in soil. J Bacteriol. 1968 Jun;95(6):1987–1992. doi: 10.1128/jb.95.6.1987-1992.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer R. F., Tiedje J. M. Application of the fluorescent-antibody technique to the study of a methanogenic bacterium in lake sediments. Appl Environ Microbiol. 1978 Jan;35(1):192–198. doi: 10.1128/aem.35.1.192-198.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Nelson D. R., Klevickis S. C., Zeikus J. G. Association of hydrogen metabolism with methanogenesis in Lake Mendota sediments. Appl Environ Microbiol. 1977 Feb;33(2):312–318. doi: 10.1128/aem.33.2.312-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Microbial methanogenesis and acetate metabolism in a meromictic lake. Appl Environ Microbiol. 1979 Feb;37(2):213–221. doi: 10.1128/aem.37.2.213-221.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Winfrey M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl Environ Microbiol. 1976 Jan;31(1):99–107. doi: 10.1128/aem.31.1.99-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



