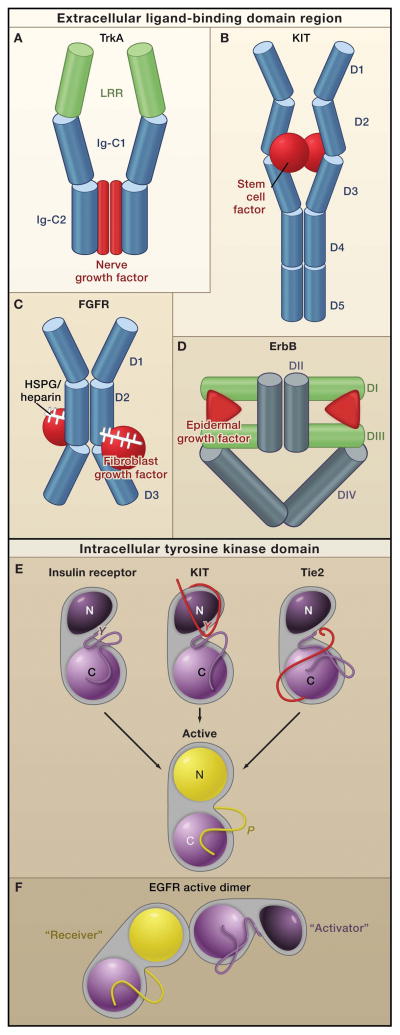
Figure 2. Receptor Tyrosine Kinase Dimerization and Kinase Activation.
Top: In general, receptor tyrosine kinases (RTKs) associate into dimers when ligand (red) binds to their extracellular regions. The bound ligand, which can form all, a portion, or none of the dimer interface, activates the receptors by stabilizing a specific relationship between two individual receptor molecules.
A. A nerve growth factor dimer (red) cross-links two TrkA molecules without any direct contact between the two receptors (Wehrman et al. 2007). B. A stem cell factor dimer (red) also cross links two KIT molecules. In addition, two Ig-like domains (D4 and D5), which reorient upon receptor activation, interact across the dimer interface (Yuzawa et al., 2007). Thus, KIT combines ligand-mediated and receptor mediated dimerization modes. C. Two fibroblast growth factor receptor (FGFR) molecules contact one another through the Ig-like domain D2, and the accessory molecule heparin or heparin sulfate proteoglycans (white sticks) also contacts this domain (Schlessinger et al., 2000). In addition, each fibroblast growth factor molecule (red) contacts Ig-like domains D2 and D3 of both FGFR molecules. D. Dimerization of ErbB receptors is mediated entirely by the receptor. Binding simultaneously to two sites (DI and DIII) within the same receptor molecule, the ligand drives conformational changes in epidermal growth factor receptor (EGFR) that expose a previously-occluded dimerization site in Domain II
Bottom: Dimerization of the extracellular regions of RTKs activates the intracellular tyrosine kinase domains (TKDs), which contain a C-lobe (light purple or yellow), N-lobe (dark purple or yellow in the inactive and active states), and an activation loop (dark purple or yellow in the inactive and active states, respectively). Although the crystal structures of the activated TKDs are very similar (Huse and Kuriyan, 2002), structures of inactive TKDs differ substantially among the receptors (top row), reflecting the diversity in their regulatory mechanisms. However, many receptors are inhibited by a set of intramolecular (or cis) interactions:
E. Insulin receptor-like (activation loop inhibition). In FGFR, insulin receptor, and IGF1-receptor the activation loop interacts directly with the active site of the kinase and blocks access to protein substrates (in FGFR) or to both ATP and protein substrates (in insulin and IGF1 receptors). Phosphorylation of key tyrosines (‘Y’) disrupts these autoinhibitory interactions and allows the kinase to ‘relax’ to the Active state.
KIT-like (juxtamembrane inhibition). In KIT, PDFGR, and Eph receptors the juxtamembrane region (red) interacts with elements within the active site of the kinase (including the αC helix and the activation loop) to stabilize an inactive conformation. Phosphorylation of key tyrosines in the juxtamembrane region destabilizes these autoinhibitory interactions and allows the TKD to assume an active conformation.
Tie1-like (C-terminal tail inhibition). In Tie1 and Tie2(and possibly Met and Ron), the C-terminal tail (red) interacts with the active site of the TKD to stabilize an inactive conformation(Shewchuk et al., 2000).
F. The EGFR TKD is allosterically activated by direct contacts between the C-lobe of one TKD, the ‘Activator,’ and the N-lobe of another TKD, ‘Receiver’ (Zhang et al., 2006). The Activator TKD destabilizes autoinhibitory interactions that involve the activation loop of the Receiver TKD. No activation loop phosphorylation is required in this mechanism (Jura et al., 2009; Red Brewer et al., 2009).