Abstract
Musical mnemonics have a long and diverse history of popular use. In addition, music processing in general is often considered spared by the neurodegenerative effects of Alzheimer’s disease (AD). Research examining these two phenomena is limited, and no work to our knowledge has explored the effectiveness of musical mnemonics in AD. The present study sought to investigate the effect of music at encoding on the subsequent recognition of associated verbal information. Lyrics of unfamiliar children’s songs were presented bimodally at encoding, as visual stimuli accompanied by either a sung or a spoken recording. Patients with AD demonstrated better recognition accuracy for the sung lyrics than the spoken lyrics, while healthy older adults showed no significant difference between the two conditions. We propose two possible explanations for these findings: first, that the brain areas subserving music processing may be preferentially spared by AD, allowing a more holistic encoding that facilitates recognition, and second, that music heightens arousal in patients with AD, allowing better attention and improved memory.
Keywords: Alzheimer’s disease, learning and memory, music, mnemonics, dementia, episodic memory
1. Introduction
Alzheimer’s disease (AD), the most common form of dementia, is characterized by a general, progressive decline in cognitive function that typically presents first as impaired episodic memory. The onset and rate of this decline tends to vary across cognitive domains, and some functions may be preferentially spared in patients with AD. Many patients, families, and caregivers consider music – or the ability to play, remember, learn, or otherwise benefit from a song – one of these rare cases in which general skill and memory are preserved in spite of otherwise severe overall impairment. The subjective reports of preserved musical processing are not limited to procedural memory, and often include stories of music used as an effective mnemonic device. For example, the daughter of an AD patient known to the authors described successfully teaching her father current events by singing news stories to the tune of popular songs (personal communication, N.R.S.). Despite the widespread informal use of music as a memory enhancer in both patient and general populations, such anecdotal reports have not received adequate empirical investigation.
Research examining general musical memory in patients with AD is limited. Several case reports present patients with moderate to severe AD who demonstrate vast general cognitive impairment but who are able to learn and play novel songs (Crystal, Grober, & Masur, 1989; Fornazzari, et al., 2006) or recognize, reproduce, and remember familiar songs (Cuddy & Duffin, 2005; Vanstone et al., 2009). Others report impaired memory for music in patients with AD (Bartlett, Halpern, & Dowling, 1995; Halpern & O'Connor, 2000; Menard & Belleville, 2009; Quoniam, et al., 2003). In a review of the literature on musical memory in AD, Baird and Samson (2009) suggest that perhaps procedural memory and priming effects for musical stimuli remain intact, whereas short-term and long-term episodic memory for melodic excerpts is impaired. In addition to these empirical findings, many anecdotal accounts describe moments of extreme lucidity in patients with severe AD, often expressed as an “awakening,” in response to autobiographically salient songs (eg. Hayden, 2007). These accounts are supported by controlled group studies showing a benefit of listening to music on autobiographical memory recall (Foster & Valentine, 2001; Irish, et al., 2006) and performance on tests of cognitive function (Thompson et al., 2005).
One possible explanation for preserved musical processing in patients with AD is that the areas of the brain associated with music cognition are preferentially spared in AD (Limb, 2006; Thompson, et al., 2003). Any latent benefit of musical mnemonics may thus become more apparent in patients with AD, for whom standard mnemonic methods are insufficient.
To our knowledge, no work has directly examined the memory-enhancing effects of music for associated information in patients with AD. Additionally, the literature on musical mnemonics in healthy populations presents an equivocal assessment of the role of music as a memory aid. Some studies show improved recall of text when studied as a sung song versus a spoken passage (Calvert & Tart, 1993; Rainey & Larsen, 2002; Wallace, 1994), while others show no advantage – or in some cases, a disadvantage – of music on text recall (eg. Racette & Peretz, 2007). Nevertheless, there is evidence suggesting a strong benefit of music, including as a mnemonic device, in a variety of clinical settings (see Hurt-Thaut, 2009 for review). The mechanisms underlying successful musical mnemonics are not well understood, but may relate generally to a shared cognitive architecture for both music and linguistic processing (Patel, 2003).
With these previous studies in mind, we set out to determine whether music can enhance new learning of information in patients with Alzheimer’s disease. In our experimental design, we sought to maximize the benefit of music by testing memory for the lyrics of children’s songs with repetitive melodies. Along with the printed lyrics on a computer screen, patients with AD and healthy controls were presented with either the words spoken, or the lyrics sung with full musical accompaniment. We hypothesized that, for both patients with AD and healthy older adults, song lyrics studied with a sung recording would be better remembered than lyrics studied with a spoken recording. We further hypothesized that the relative benefit of the sung compared to the spoken condition would be greater in the patient group than in the control group.
2. Methods
2.1 Participants
Thirteen patients with a clinical diagnosis of probable AD and 14 healthy older adults were recruited for this study. Patients with probable AD met the criteria set forth by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA; McKhann, et al., 1984) and healthy older adults were defined as demonstrating no cognitive impairment on a standard battery of neuropsychological tests (Table 1) and having no first-degree relatives with a history of AD or other neurodegenerative disorders or dementias. Participants with AD were recruited from the clinical populations of the Boston University Alzheimer’s Disease Center, also in Boston, MA, and the Memory Diagnostic Clinic at the Bedford VA Hospital in Bedford, MA. Healthy controls were recruited from online and community postings in the Boston area, or were the spouses of the AD patients who participated in the study. Participants were excluded if they had a history of clinically significant depression, alcohol or drug use, cerebrovascular disease, traumatic brain injury, or if they had uncorrected vision or hearing problems.
Table 1.
Demographic and standard neuropsychological test data by group.
| OC | AD | |
|---|---|---|
| Gender | 4M/8F | 9M/3F* |
| Age | 73.7 (5.5) | 77.3 (7.6) |
| Years of Education | 16 (2.3) | 14 (3.3) |
| Musical Experience | 4Y/8N | 3Y/9N |
| MMSE | 30 (0.5) | 24 (4.6)** |
| CERAD | ||
| Immediate | 22.0 (3.7) | 11.1 (3.6)** |
| Delayed | 7.4 (2.0) | .75 (1.0)** |
| Recognition | 9.8 (.45) | 5.2 (3.3)** |
| Trails-B | 84.6 (28.3) | 237.8 (68.2)** |
| FAS | 48.8 (10.5) | 32.5 (14.7)* |
| CAT | 46 (11.0) | 26.4 (12.7)** |
| BNT-15 | ||
| No Cue | 14 (2) | 11 (3.6)* |
| Semantic Cue | 0.1 (0.3) | 0 (0) |
| Phonemic Cue | 0.7 (1.5) | 1.9 (1.6) |
Notes: Standard deviations are presented in italics. OC = healthy older adults; MMSE = Mini Mental State Examination; CERAD = CERAD Word List Memory Test; Trails-B = Trail Making Test Part B; FAS and CAT = Verbal Fluency; BNT-15 = 15-item Boston Naming Test. Musical experience was defined as having any formal instrument or voice training and was self-reported by the participant.
Significant group differences are indicated by *(p<.05) and ** (p<.005).
Each participant completed a brief neuropsychological battery in a 45-minute session either directly following the experimental session or on a different date. Table 1 presents demographic and neuropsychological data for the two groups of participants. Two control subjects were excluded because of overall impaired performance on these tests, and one patient was excluded because of a failure to understand the task instructions.
Independent-samples t-tests revealed no significant differences between groups in age [t(22)=1.323, p= .200)], years of education [t(22)=−1.662, p=.111], or self-reported musical experience [t(22)=−.842, p=.409]. However, there was a significant difference in gender [t(22),=−2.159, p=.042] between groups, with nine males in the AD group versus only four in the control group.
The human subjects committee of the Bedford VA Hospital approved this study, and written informed consents were obtained from all participants and their caregivers, where appropriate. Participants were compensated $10/hr for their participation.
2.2 Stimuli
Stimuli consisted of four-line excerpts of eighty children’s songs gathered from the KIDiddles online children’s music database (http://www.kididdles.com) and determined to be wholly unfamiliar by a pre-screening survey. Songs were selected to have simple, unrepeated lyrics, repetitive melodies, and a perfect tail rhyme scheme for the four lines used.
A sung version and a spoken version of each of the eighty song excerpts were created in Apple’s Logic Pro 8 (Version 8.0.2; Apple Inc.). A 17-year-old female vocalist with extensive musical and voice training was recruited to sing and speak all song excerpts. Multi-track instrumental MIDI recordings for the songs were obtained with permission from KIDiddles, and the sung recordings of each track consisted of the sung vocal track accompanied by these instrumentals. Spoken excerpts were recorded with normal vocal inflection at the same speed as their corresponding sung excerpt.
The eighty songs were organized into four lists counterbalanced, in order of priority, for total words (M=22.64, SD=4.83), sung recording length (M=26.45s, SD=7.0852), spoken recording length (M=27.10s, SD=6.84), Flesch-Kincaid Grade Level, or the expected years of education necessary for text comprehension (M=4.12, SD=2.06), and Flesch Reading Ease, a related measure of text readability (M=86.77, SD=10.38). The presentation condition was counterbalanced across subject so that each song appeared an equal number of times in each condition.
2.3 Design and Procedure
Participants were tested individually in a single session lasting approximately 30 minutes. Stimuli were presented on a Dell Inspiron 640m laptop computer via E-Prime software, version 2.0 (Psychology Software Tools Inc.; www.pst-net.com/eprime). During the study phase, participants were presented visually with the lyrics to 40 songs. The four-line lyrics were center justified and displayed in their entirety for the duration of stimulus presentation. Twenty of the song lyrics were accompanied by their corresponding sung recording and 20 were accompanied by their spoken recording. Stimuli were presented at random with respect to condition. Each recording repeated two times, consecutively. Audio recordings were presented via over-ear ATH-M30 Professional headphones by Audio-Technica (http://www.audio-technica.com) and, in the absence of an objective measure of auditory acuity, participants were asked during an example study-test phase to adjust the volume to a comfortable and clearly audible level. The minimum selected headphone volume resulted in stimuli presentation at between 60 and 65 decibels (dB) and the maximum selected volume resulted in presentation at between 80 and 85 dB. Participants were informed that their memory for the lyrics would be tested, and were asked after each presentation to indicate whether or not they were previously familiar with the song they had just heard. During the test phase, the lyrics to all 80 songs were presented without any audio recording and participants were asked to make an “old or new?” recognition judgment.
Despite the pre-screening familiarity survey, some participants reported knowledge of certain songs during the study phase of the experiment. For controls, these songs were excluded from the data analyses. For patients with AD, the familiarity judgments appeared to be too inconsistent and frequent to indicate any real prior knowledge of the songs and were therefore disregarded in the main analyses. Another set of analyses was conducted, however, which excluded all these songs deemed familiar to the AD patients. These secondary analyses showed a very similar pattern to those of the main analyses, suggesting that the familiarity judgments of the AD patients, real or false, did not affect the findings of this study. Additionally, one song was reported as familiar by six of the twelve healthy participants and was excluded from the data for all participants in both groups.
3. Results
Our main analyses of interest were to evaluate the difference in memory performance between the sung and spoken conditions for healthy older adults and patients with AD. We performed an ANOVA using the accuracy measure Pr (%hits - %false alarms; Snodgrass & Corwin, 1988). Because of group differences, gender was included as a between-subjects factor in the initial analysis. This analysis revealed no effects of or interactions with gender. The data were therefore re-analyzed without gender as a factor, and the results of this analysis are presented.
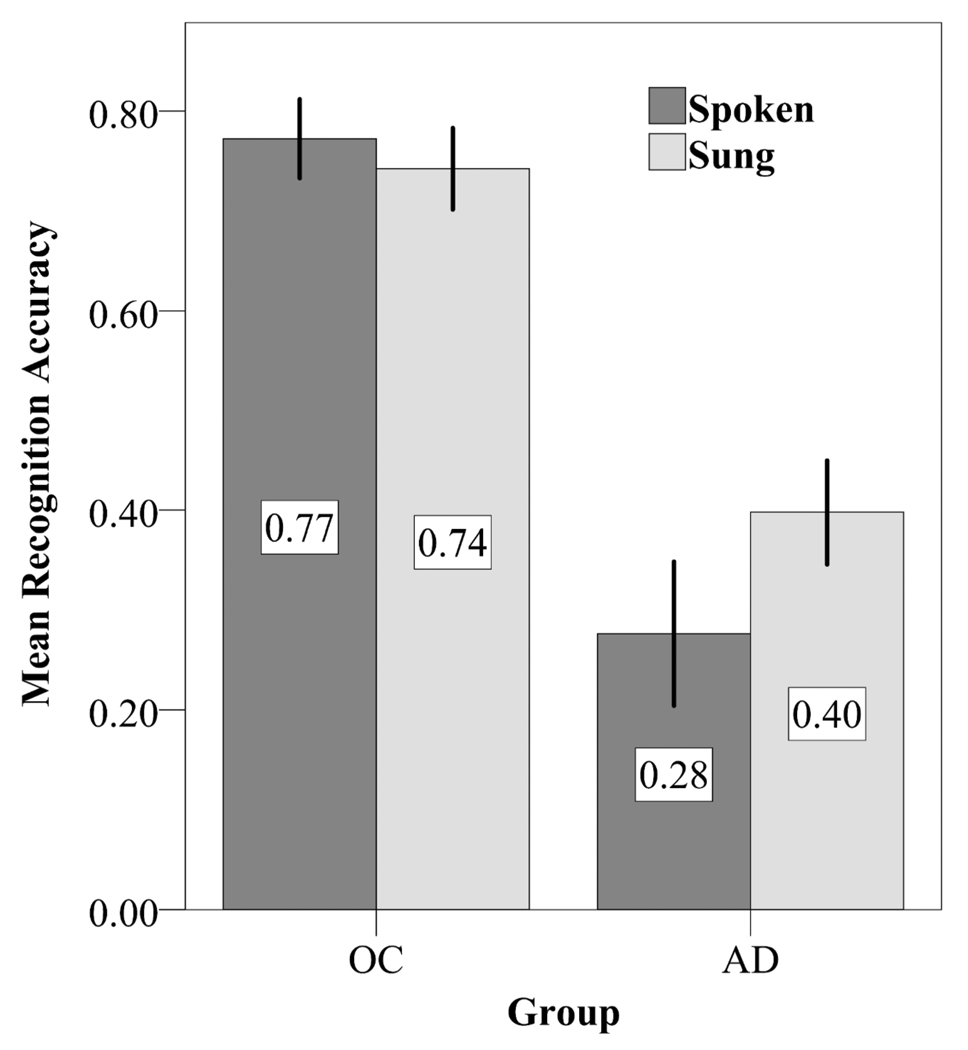
To evaluate accuracy (Pr), we performed a repeated measures ANOVA with the factors of Group (AD, healthy older adults) and Condition (sung, spoken). The ANOVA revealed main effects of Group [F(1,22)=32.72, p<.001], as healthy older adults performed better on the recognition task than patients with AD, and Condition [F(1,22)=5.68, p=.026], as Pr was better for the sung condition (.58) than for the spoken condition (.53) across groups. In addition to these main effects, the ANOVA revealed a Group by Condition interaction [F(1,22)=15.62, p=.001]. To investigate the interaction, paired-sample t-tests were performed. These revealed that Pr was greater in the sung condition than in the spoken condition for AD patients [t(11)=3.868, p=.003] but not for healthy older controls [t(11)=1.325, p=.212] (see Figure 1).
Fig. 1.
Mean recognition accuracy (Pr; %hits - %false alarms) for the sung and spoken conditions in healthy older adults (OC) and patients with Alzheimer's disease (AD). Error bars represent one standard error of the mean.
4. Discussion
The main goal of the present study was to determine the extent to which music can be used to enhance memory for associated verbal information in patients with AD and healthy older adults. The results confirmed our hypothesis that patients with AD performed better on a task of recognition memory for the lyrics of songs when those lyrics were accompanied at encoding by a sung recording than when they were accompanied by a spoken recording. However, contrary to our hypothesis, healthy older adults showed no such benefit of music.
These results suggest a fundamental difference in the encoding and retrieval processes for musical versus nonmusical stimuli between patients with AD and healthy older adults. One possible explanation for the observed dissociation in benefit of song is that in patients, where general cortical and hippocampal atrophy impair standard episodic learning, musically-associated stimuli allow for a more diversified encoding. Music processing encompasses a complex neural network that recruits from all areas of the brain, including subcortical areas such as the basal ganglia, nucleus accumbens, ventral tegmental area, hypothalamus, and cerebellum (Grahn, 2009; Levitin & Tirovolas, 2009; Limb, 2006) and cortical areas such as the medial prefrontal cortex (Janata, 2009) and orbitofrontal cortex (Limb, 2006) that are affected at a slower rate in AD compared to the areas of the brain typically associated with memory (Thompson et al., 2003). Thus, stimuli accompanied by music and a sung recording may create a more robust association at encoding than do stimuli accompanied by only a spoken recording in patients with AD. Additionally, musical mnemonics have been shown to induce oscillatory synchrony in neural networks associated with verbal learning and memory (Thaut, Peterson, & McIntosh, 2005), and such synchronous neuronal firing may support this more composite encoding and retrieval process. Musical mnemonics provide an intricate neurophysiologic template for the mapping of verbal information in temporal and tonal space, and this template may be used during retrieval to aid in lyric recognition or recall. For healthy older adults with intact cortical memory circuits, it is possible that the relatively simple nature of the recognition task in the current paradigm does not produce a need to recruit from these areas of the brain not typically used for recognition memory, and so results in no difference between the sung and spoken conditions. In other words, there may be no need to rely on the holistic musical memory “backup” at the part of the performance scale where control subjects scored.
It is also possible that attentional deficits in patients with AD (see Perry & Hodges, 1999 for review), and the ability of music to moderate these deficits by heightening arousal (Thompson, Schellenberg, & Husain, 2001), account for the effect of condition in the patient group. Here the affective nature of the song stimuli in the present experiment deserves further consideration. The pleasant, upbeat quality of the children’s songs chosen might have enhanced concentration during sung stimuli presentation, resulting in more focused encoding and improved recognition. Again, for healthy older adults without any attentional deficits, the current study may not represent a situation in which the benefit of music as an attentional aid is necessary.
Future experiments can explore these two possibilities more thoroughly by manipulating task difficulty to eliminate general scaling effects and limiting available attentional resources in controls to normalize ability between groups. To examine the lingering effects of music-induced arousal on subsequent non-musical stimuli, follow-up work might additionally control for stimulus presentation order. It will also be important to better understand the specific qualities of an effective musical mnemonic. For example, does the benefit of song in patients remain if the lyrics are sung without any instrumental accompaniment?
Lastly, our findings offer many possible applications in the treatment of and care for patients with AD. The multimodal nature of the studied stimuli in the present paradigm and the unimodal design of the recognition test suggest that it may be possible to use musical mnemonics to teach novel information to patients with AD, and that this information may be retrieved across modalities. Future research can examine, for example, the possibility of presenting practical, every-day information to patients with AD in the form of song. Understanding the nature of musical processing and memory in patients with AD may allow the development of effective and comprehensive therapies for this increasingly prevalent disease.
Table 2.
Hit and false alarm rates by group
| OC | AD | |
|---|---|---|
| Spoken Hits | 0.84 (0.10) | 0.56 (0.25) |
| Sung Hits | 0.81 (0.09) | 0.68 (0.24) |
| Overall Hits | 0.82 (0.09) | 0.62 (0.24) |
| False Alarms | 0.14 (0.09) | 0.28 (0.23) |
Notes: Standard deviations are presented in italics. OC = healthy older adults. Overall hits reported as the average hit rate (% hits) for stimuli studied both sung (sung hits) and spoken (spoken hits).
Acknowledgments
This research was supported by National Institute on Aging grants P30 AG13846, R01 AG 025815 (AEB), and K23 AG031925 (BAA). This material is also the result of work supported with resources and the use of facilities at the Edith Nourse Rogers Memorial Veterans Hospital in Bedford, MA. We would like to thank Anna Miller for providing the vocals for our stimuli and KIDiddles for allowing use of its MIDI recordings.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baird A, Samson S. Memory for music in Alzheimer's disease: unforgettable? Neuropsychol Rev. 2009;19(1):85–101. doi: 10.1007/s11065-009-9085-2. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Halpern AR, Dowling WJ. Recognition of familiar and unfamiliar melodies in normal aging and Alzheimer's disease. Mem Cognit. 1995;23(5):531–546. doi: 10.3758/bf03197255. [DOI] [PubMed] [Google Scholar]
- Calvert SL, Tart M. Song Versus Verbal Forms for Very-Long-Term, Long-Term, and Short-Term Verbatim Recall. Journal of Applied Developmental Psychology. 1993;14:245–260. [Google Scholar]
- Crystal HA, Grober E, Masur D. Preservation of musical memory in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1989;52(12):1415–1416. doi: 10.1136/jnnp.52.12.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddy LL, Duffin J. Music, memory, and Alzheimer's disease: is music recognition spared in dementia, and how can it be assessed? Medical Hypotheses. 2005;64(2):229–235. doi: 10.1016/j.mehy.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Fornazzari L, Castle T, Nadkarni S, Ambrose M, Miranda D, Apanasiewicz N, et al. Preservation of episodic musical memory in a pianist with Alzheimer disease. Neurology. 2006;66(4):610–611. doi: 10.1212/01.WNL.0000198242.13411.FB. [DOI] [PubMed] [Google Scholar]
- Foster NA, Valentine ER. The effect of auditory stimulation on autobiographical recall in dementia. Exp Aging Res. 2001;27(3):215–228. doi: 10.1080/036107301300208664. [DOI] [PubMed] [Google Scholar]
- Grahn JA. The role of the basal ganglia in beat perception: neuroimaging and neuropsychological investigations. Ann N Y Acad Sci. 2009;1169:35–45. doi: 10.1111/j.1749-6632.2009.04553.x. [DOI] [PubMed] [Google Scholar]
- Halpern AR, O'Connor MG. Implicit memory for music in Alzheimer's disease. Neuropsychology. 2000;14(3):391–397. doi: 10.1037//0894-4105.14.3.391. [DOI] [PubMed] [Google Scholar]
- Hayden JS. Some enchanted evening … like magic, a familiar song conjures up memories of a happier time. Nursing. 2007;37(10):2. doi: 10.1097/01.NURSE.0000291988.01314.77. [DOI] [PubMed] [Google Scholar]
- Hurt-Thaut C. Clinical practice in music therapy. In: Hallam S, Cross I, Thaut MH, editors. The Oxford Handbook of Music Psychology. New York, NY: Oxford University Press; 2009. pp. 503–514. [Google Scholar]
- Irish M, Cunningham CJ, Walsh JB, Coakley D, Lawlor BA, Robertson IH, et al. Investigating the enhancing effect of music on autobiographical memory in mild Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;22(1):108–120. doi: 10.1159/000093487. [DOI] [PubMed] [Google Scholar]
- Janata P. The Neural Architecture of Music-Evoked Autobiographical Memories. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitin DJ, Tirovolas AK. Current advances in the cognitive neuroscience of music. Ann N Y Acad Sci. 2009;1156:211–231. doi: 10.1111/j.1749-6632.2009.04417.x. [DOI] [PubMed] [Google Scholar]
- Limb CJ. Structural and functional neural correlates of music perception. Anat Rec A Discov Mol Cell Evol Biol. 2006;288(4):435–446. doi: 10.1002/ar.a.20316. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Menard MC, Belleville S. Musical and verbal memory in Alzheimer's disease: a study of long-term and short-term memory. Brain Cogn. 2009;71(1):38–45. doi: 10.1016/j.bandc.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Patel AD. Language, music, syntax and the brain. Nat Neurosci. 2003;6(7):674–681. doi: 10.1038/nn1082. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer's disease. A critical review. Brain. 1999;122(Pt 3):383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Quoniam N, Ergis AM, Fossati P, Peretz I, Samson S, Sarazin M, et al. Implicit and explicit emotional memory for melodies in Alzheimer's disease and depression. Ann N Y Acad Sci. 2003;999:381–384. doi: 10.1196/annals.1284.047. [DOI] [PubMed] [Google Scholar]
- Racette A, Peretz I. Learning lyrics: To sing or not to sing? Memory & Cognition. 2007;35(2):242–253. doi: 10.3758/bf03193445. [DOI] [PubMed] [Google Scholar]
- Rainey DW, Larsen JD. The Effect of Familiar Melodies on Initial Learning and Long-term Memory for Unconnected Text. Music Perception. 2002;20(2):173–186. [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Thaut MH, Peterson DA, McIntosh GC. Temporal entrainment of cognitive functions: musical mnemonics induce brain plasticity and oscillatory synchrony in neural networks underlying memory. Ann N Y Acad Sci. 2005;1060:243–254. doi: 10.1196/annals.1360.017. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RG, Moulin CJ, Hayre S, Jones RW. Music enhances category fluency in healthy older adults and Alzheimer's disease patients. Exp Aging Res. 2005;31(1):91–99. doi: 10.1080/03610730590882819. [DOI] [PubMed] [Google Scholar]
- Thompson WF, Schellenberg EG, Husain G. Arousal, mood, and the Mozart effect. Psychol Sci. 2001;12(3):248–251. doi: 10.1111/1467-9280.00345. [DOI] [PubMed] [Google Scholar]
- Vanstone AD, Cuddy LL, Duffin JM, Alexander E. Exceptional Preservation of Memory for Tunes and Lyrics Case Studies of Amusia, Profound Deafness, and Alzheimer's Disease. Neurosciences and Music Iii: Disorders and Plasticity. 2009;1169:291–294. doi: 10.1111/j.1749-6632.2009.04763.x. [DOI] [PubMed] [Google Scholar]
- Wallace WT. Memory for Music - Effect of Melody on Recall of Text. Journal of Experimental Psychology-Learning Memory and Cognition. 1994;20(6):1471–1485. [Google Scholar]