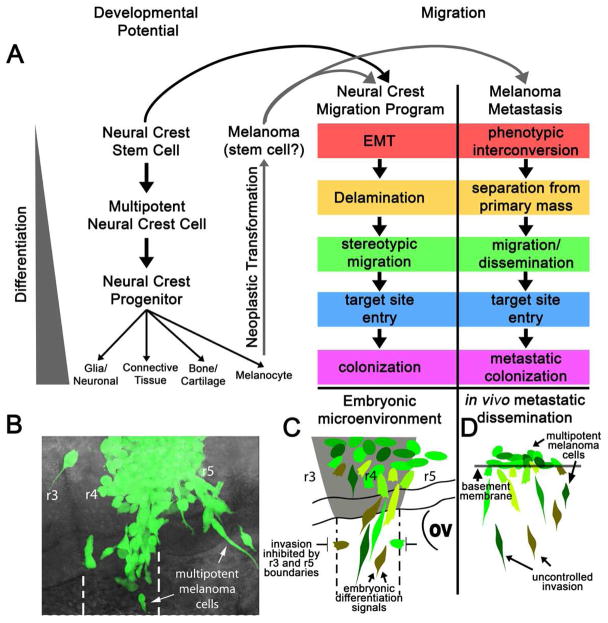
Figure 3. Common features of the multipotent neural crest cell and neural crest-derived cancer cell metastatic program.
The neural crest migration program shares many similarities to melanoma metastasis. (A) A cartoon depicting the neural crest migration program and developmental potential. Neural crest stem cells give rise to a multipotent neural crest cell population that emigrates to a specific, defined site of differentiation and gives rise to diverse cell types including pigment cells. Following neoplastic transformation, melanocytes display many stem-cell-like traits, suggesting that melanoma cells reacquire specific neural crest attributes. The neural crest migratory program parallels many aspects of melanoma metastasis, and when aggressive human melanoma cells are transplanted into the chick embryonic neural crest microenvironment, they exhibit behaviors typical of neural crest migration. (B) GFP-labeled c8161 human melanoma cells transplanted into the chick neural tube at the rhombomere 4 (r4) axial level exit the dorsal neural tube and migrate along the r4 neural crest migratory pathway while generally avoiding the NC-free zones. (C) The schematic shows that human melanoma cells respect the host embryonic neural crest cell-free zones adjacent to r3 and r5, and a subset of the invading human melanoma cells may be influenced by the host embryonic neural crest microenvironment to express genes characteristic of a neural crest-like phenotype (data in Kulesa et al., 2006). The neural tube region of r4 and the boundaries between the host r4 NCC migratory stream and neural crest cell-free zones are highlighted. (D) In comparison, a schematic representation of in vivo metastatic dissemination highlights the unprogrammed invasion of NC-derived tumor cells in the human microenvironment.