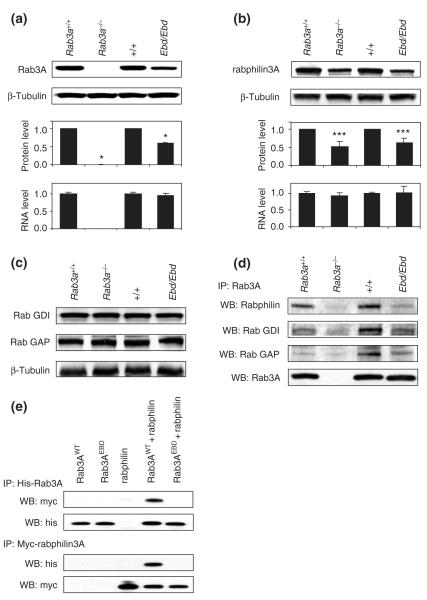
Figure 2. Protein stability of ras-associated binding (Rab) protein 3A and its effectors and their interactions in mutant mice.
(a) Rab3A protein levels were examined by immunoblotting with anti-Rab3A in homogenates from the hippocampus of earlybird (Ebd) and Rab3a null mice. The upper two panels show the representative Western blots for indicated wild-type and mutant strains of mice. The protein levels were quantified using ImageJ and normalized to β-tubulin. The mRNA levels of Rab3A measured by real-time quantitative polymerase chain reaction. Data are means ± SD. *P < 0.001, student’s t-test. (b) Rabphilin3A protein levels (upper and middle panels) and mRNA levels (lower) in the hippocampus of Ebd and Rab3a-null mice were examined similarly as described in (a). (c) Protein levels of Rab GDI and Rab GAP in the cortex of Ebd and Rab3a-null mice were examined by Western blot using anti-Rab GDI1 and anti-Rab GAP p130 antibodies. Beta-tubulin was also examined as a control for sample input. (d) Co-immunoprecipitation in the cortex homogenates. Rab3A was immunoprecipitated with anti-Rab3A antibodies, and Rab3A-bound rabphilin3A, Rab GDI and Rab GAP were detected using respective antibodies in Western blot. Anti-Rab3A antibodies were used to examine the sample input (lower panel). (e) Co-immunoprecipitation in the lysates of HEK 293 cells co-transfected with His-tagged Rab3A and Myc-tagged rabphilin3A.