Abstract
Four stereoisomers of the C21–C40 fragment are synthesized in a single exercise with the aid of fluorous tagging to encode configurations at C37 and C33. After demixing and detagging, the isomers were found to have substantially identical 1H NMR spectra. However, there were some small but reliable differences in their 13C NMR spectra.
Keywords: tetrafibricin, fibrinogen, fluorous mixture synthesis, quasiisomers, Kocienski-Julia reaction
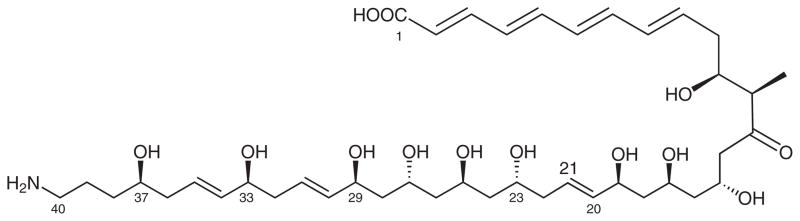
In 1993, Kamiyama and co-workers described the isolation and two-dimensional structure (constitution) of the interesting natural product tetrafibricin (1, Figure 1).1 The compound is an ω-amino acid whose terminal groups are separated by 39 carbon atoms. Adding the carboxylate, the total length of the carbon backbone is 40 atoms. The chain features seven E-alkenes, ten hydroxy groups, a ketone, and a lone methyl group.
Figure 1.
Proposed structure of tetrafibricin (1)
Tetrafibricin strongly inhibited the binding of fibrinogen to its glycoprotein receptor (IC50 = 46 nM). It also inhibited ADP-, collagen-, and thrombin-induced aggregation of human platelets.1 Accordingly, it has potential as a therapeutic agent for arterial thrombotic diseases.
Assignment of the three-dimensional structure (configuration) of tetrafibricin is a problem because the stereocenters or groups of stereocenters are insulated from each other by 2–3 chain carbon atoms. Kishi and co-workers addressed the problem without using either classical derivatization or degradation by comparing NMR data of the natural product and its ketone reduction products collected in both chiral and achiral solvents to values in a database built from spectra of suitable model compounds.2
It would be valuable to confirm the structure of tetrafibricin by total synthesis. Syntheses of various fragments and assorted couplings have been described by Cossy3a and Roush3b as well as by our group.3c,d We are also interested in making stereoisomers of tetrafibricin both to flesh out SAR and to learn whether and how the isomers can be differentiated. Towards these ends, we describe herein the synthesis of four stereoisomers of a large bottom fragment of tetrafibricin. We use the technique of fluorous mixture synthesis4 to make all four isomers together in a single synthetic sequence.
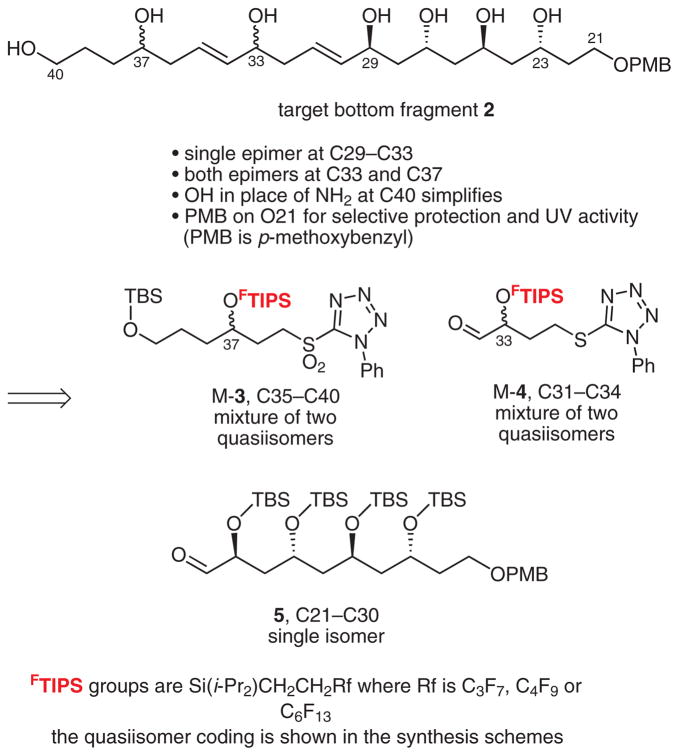
The retrosynthetic analysis of tetrafibricin breaks the molecule at the C20–C21 alkene into large top and bottom fragments of comparable size and complexity. Further analysis of the bottom fragment 2 is shown in Scheme 1. This has two stereocenters (C33 and C37) that are isolated from others by three atoms along with a group of four stereocenters (C23–C29) on alternating carbon atoms.
Scheme 1.
Retrosynthetic analysis of bottom fragment 2
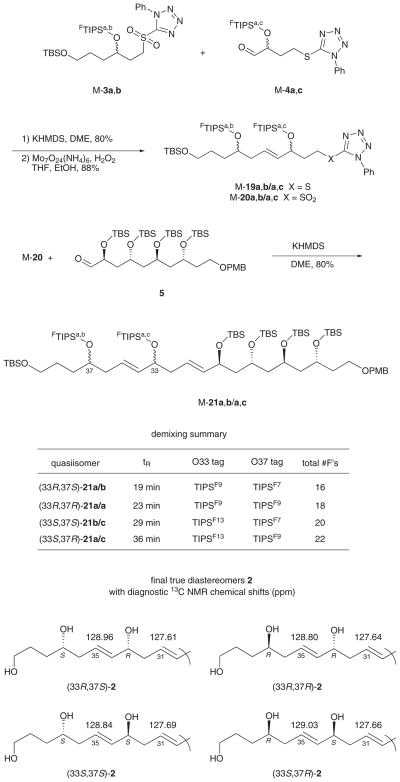
Accordingly, coupling of 3, 4, and 5 by Kocienski–Julia reactions5 should provide the bottom fragment 2 with flexible access to various isomers. To make the set of four possible diastereomers that these three isomer groups engender, we decided to fix the absolute configuration of the large fragment 5 while making small fragments 3 and 4 as quasiisomer mixtures4e with component configurations encoded by fluorous tags (mixture samples are denoted by the prefix ‘M’). This fluorous mixture synthesis was patterned after a single isomer synthesis by Dr. V. Gudipati.3c
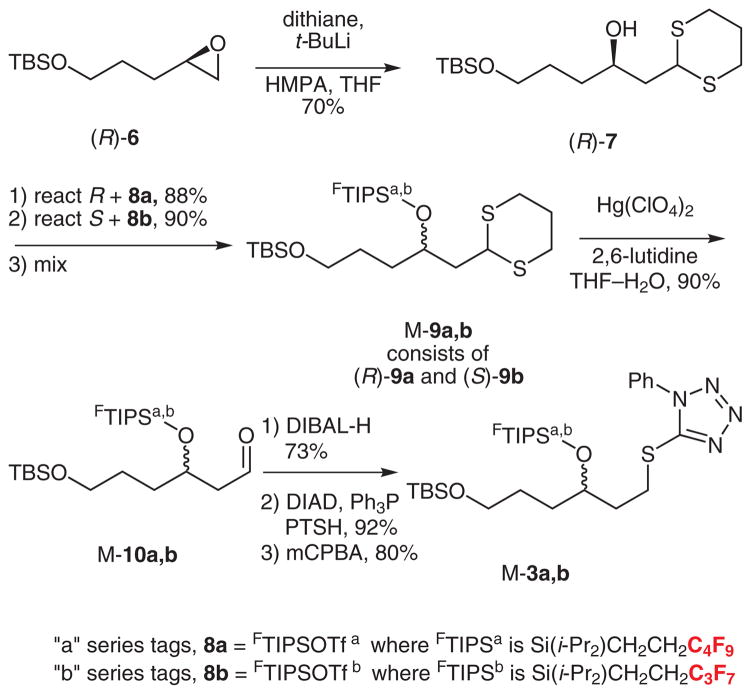
The synthesis of quasiracemate M-3 is summarized in Scheme 2. Epoxide (R)-6 was prepared by a Jacobsen hydrolytic kinetic resolution (HKR),6 then opened with lithiodithiane to give (R)-7 in 70% yield.7 Likewise, (S)-7 was prepared from (S)-6 (not shown). The configurations at C37 were encoded by silylation with fluorous silyl tri-flates; (R)-7 was reacted with 8a bearing a C4F9 substituent while (S)-7 was reacted with 8b bearing a C3F7 substituent.8 The resulting quasienantiomers (R)-9a/(S)-9b (88% and 90% yields) were mixed in equal amounts to make a quasiracemate M-9a,b, then the dithiane was carefully hydrolyzed with mercuric perchlorate to provide M-10a,b (90%).9
Scheme 2.
Synthesis of sulfone quasiracemate M-3
Reduction of M-10a,b with DIBAL-H (73%), Mitsunobu reaction10 of the resulting alcohol with 1-phenyl-1H-tetra-zole-5-thiol (PTSH, 92%), and finally mCPBA oxidation (80%) provided the target sulfone component M-3a,b for first Kocienski–Julia coupling.
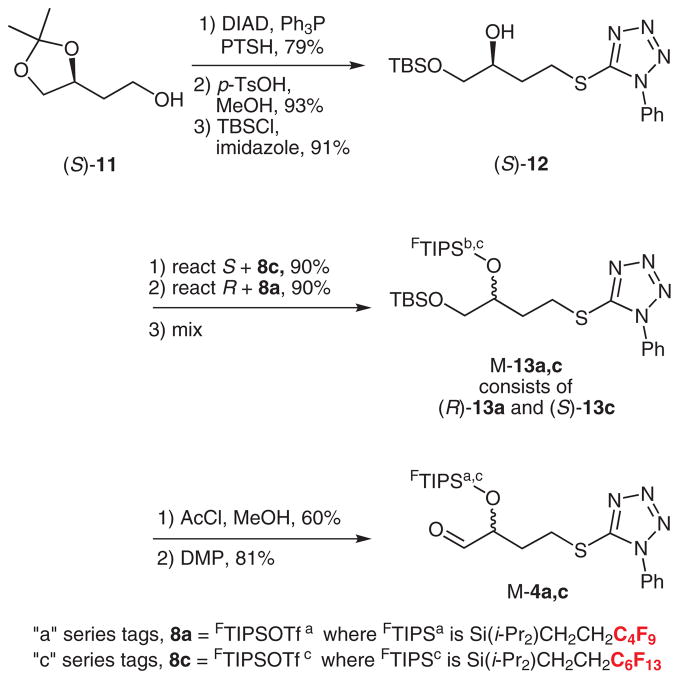
The synthesis of the aldehyde component for first coupling is shown in Scheme 3. Coupling of alcohol (S)-11 with PTSH as above (79%) followed by hydrolysis (93%) and selective silylation of the primary alcohol with TBSCl (91%) provided (S)-12. Likewise, (R)-12 was made by the same sequence of reactions starting from (R)-11.8
Scheme 3.
Synthesis of aldehyde quasiracemate M-4
The S-enantiomer was tagged with silyl triflate bearing a C6F13 group to give (S)-13c. In turn quasienantiomer (R)-13a with a C4F9 group was made from (R)-12 and 8a. Both silylations occurred in 90% yield.
These quasienantiomers were mixed in equal portions to give quasiracemate M-13a,c. The TBS group was selectively removed by careful hydrolysis with HCl in methanol (60%), and the resulting alcohol was oxidized with Dess–Martin reagent to give M-4a,c (81%, Scheme 3).11
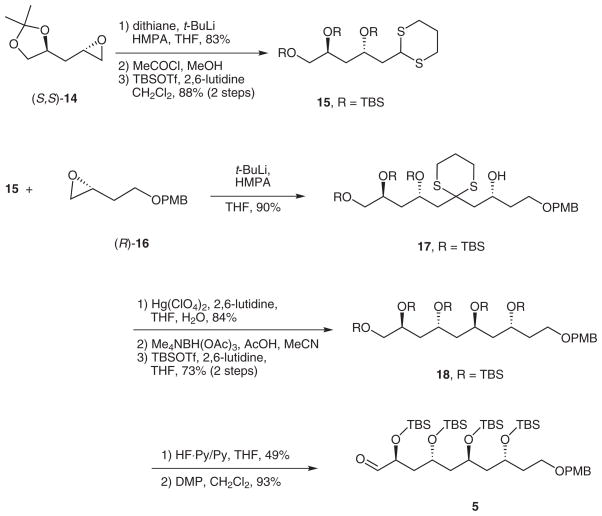
The synthesis of the single enantiomer of the C21–C30 fragment 5 is summarized in Scheme 4. Epoxide (S,S)-14 was prepared by Jacobsen HKR,6 then opened with lithiodithiane (83%).7 Hydrolysis of the acetal followed by protection of the resulting triol with TBSOTf gave trissilyl ether 15 (88% over two steps).
Scheme 4.
Synthesis of single isomer fragment 5
Deprotonation of 15 with t-BuLi and reaction of the derived dithiane anion with epoxide (R)-16 provided 17 in 90% yield. Dithiane hydrolysis (84%) followed by directed reduction provided a 1,3-anti-diol trissilyl ether12 that was further protected with TBSOTf to provide pentakissilyl ether 18 (73% for two steps). Selective desilylation of the primary TBS ether with HF·pyridine13 occurred in 49% yield, then Dess–Martin oxidation11 provided aldehyde 5 as a single isomer.
The fragment couplings and completion of the synthesis of the four isomers of 2 were all conducted in fluorous mixture mode, as summarized in Scheme 5. Kocienski–Julia coupling of quasiracemates M-3a,b and M-4a,c with KHMDS in DME provided a mixture of four quasiisomers M-19 with an E/Z selectivity of about 9:1. Furthermore, we discovered that we could separate the minor Z-isomers without demixing any of the quasiisomers by preparative HPLC on a Whelk-O1 column. Thus, even though the crude Kocienski–Julia product contained eight true isomers (E/Z) and quasiisomers combined, the chromatogram exhibited only two peaks; a major one consisting of the four E-quasiisomers (M-19) and a minor one of the four Z-quasiisomers.
Scheme 5.
Fragment coupling, demixing, and detagging
Oxidation of the purified M-19 provided sulfone M-20,14 which was them coupled with 5 as above to provide alkene M-21. Again this was a mixture of four quasiisomers, each which was present as about a 9:1 mixture of E/Z isomers. Yet again preparative HPLC separated the four minor Z-quasiisomers without demixing the major E-isomers.
The mixture of four E,E-quasiisomers M-21 was readily demixed into its individual components by preparative HPLC over a Fluoroflash15 column. A representative HPLC trace of a preparative run is shown in Figure S1 of the Supporting Information, and a summary of the product structures with configurations and tags is shown in Scheme 5. Final detagging to give the true isomers of 2 proved difficult because of the polarity of the product. However, desilylation with TASF in DMF4b followed by solvent removal and direct purification provided each of the four individual isomers in pure form in about 75% yield.
An important goal of this work was to learn whether the four diastereomers of 2 could be differentiated from each other by NMR spectroscopy. Copies of the 1H NMR and 13C NMR spectra of the four pure isomers of 2 are provided in the Supporting Information. The four 1H NMR spectra in CDCl3 at 700 MHz were substantially identical. In other words, none of the four isomers of 2 could be differentiated from any other by this means.
The four 13C NMR spectra (CDCl3 at 175 MHz) were very similar, but not identical. In particular, the 33,37-anti isomers (R,R and S,S) could be differentiated from the syn isomers (R,S and S,R) by the chemical shift of alkene carbon 35, which was below δ = 128.90 ppm for the anti isomers and above δ = 128.90 ppm for the syn isomers. Chemical shift differences for C35 of the syn/anti isomers range from 0.12–0.23 ppm. Though small, these differences are probably reliable. Oishi and co-workers have recently identified a similar effect in related substructures of amphidiniol 3, which led them to revise the structure of this natural product.16
Differentiating the pairs of C33/C37 syn and anti isomers from each other (R,R from S,S and R,S from S,R) is more difficult. However, we suggest that this can be achieved by comparing the chemical shifts of C35 (again) and C31. Here, the differences are less, 0.04–0.07 ppm, but the confidence level is increased since there are two values to compare. There are other small differences in the 13C NMR spectra, that are tabulated in the Supporting Information along with all possible isomer subtractions to facilitate comparison.
In summary, we have made four stereoisomers of a large bottom fragment of tetrafibricin and compared them to each other by 1H NMR and 13C NMR spectroscopy. Although the stereoclusters are separated from each other by only three carbon atoms, the 1H NMR spectra of the isomers are substantially identical. The 13C NMR spectra are very similar, but not completely identical. We have learned from comparison of the spectra which resonances are diagnostic for differentiating the isomers.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health, National Institute of General Medical Sciences, for funding this work.
Footnotes
Dedicated to Prof. Gerry Pattenden in celebration of his 70th birthday
Supporting Information for this article is available online at http://www.thieme-connect.com/ejournals/toc/synlett. The file contains full experimental details and compound characterizations along with copies of NMR spectra of quasiisomers 21 and diastereomers 2 and a comparison table of 13C NMR chemical shifts of 2.
References and Notes
- 1.(a) Kamiyama T, Itezono Y, Umino T, Satoh T, Nakayama N, Yokose K. J Antibiot. 1993;46:1047. doi: 10.7164/antibiotics.46.1047. [DOI] [PubMed] [Google Scholar]; (b) Kamiyama T, Umino T, Fujisaki N, Fujimori K, Satoh T, Yamashita Y, Ohshima S, Watanabe J, Yokose K. J Antibiot. 1993;46:1039. doi: 10.7164/antibiotics.46.1039. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi Y, Czechtizky W, Kishi Y. Org Lett. 2003;5:93. doi: 10.1021/ol0272895. [DOI] [PubMed] [Google Scholar]
- 3.(a) BouzBouz S, Cossy J. Org Lett. 2004;6:3469. doi: 10.1021/ol048862i. [DOI] [PubMed] [Google Scholar]; (b) Lira R, Roush WR. Org Lett. 2007;9:533. doi: 10.1021/ol0629869. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gudipati V. PhD Thesis. University of Pittsburgh; USA: 2008. http://etd.library.pitt.edu/ETD/available/etd-04212008-115104/ [Google Scholar]; (d) Gudipati V, Bajpai R, Curran DP. Collect Czech Chem Commun. 2009;74:774. doi: 10.1135/cccc2008195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Luo ZY, Zhang QS, Oderaotoshi Y, Curran DP. Science. 2001;291:1766. doi: 10.1126/science.1057567. [DOI] [PubMed] [Google Scholar]; (b) Yang F, Newsome JJ, Curran DP. J Am Chem Soc. 2006;128:14200. doi: 10.1021/ja064812s. [DOI] [PubMed] [Google Scholar]; (c) Jung WH, Guyenne S, Riesco-Fagundo C, Mancuso J, Nakamura S, Curran DP. Angew Chem Int Ed. 2008;47:1130. doi: 10.1002/anie.200704893. [DOI] [PubMed] [Google Scholar]; (d) Curran DP, Sui B. J Am Chem Soc. 2009;131:5411. doi: 10.1021/ja900849f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhang QS, Curran DP. Chem Eur J. 2005;11:4866. doi: 10.1002/chem.200500076. [DOI] [PubMed] [Google Scholar]
- 5.Blakemore PR. J Chem Soc, Perkin Trans 1. 2002:2563. [Google Scholar]
- 6.Schaus SE, Brandes BD, Larrow JF, Tokunaga M, Hansen KB, Gould AE, Furrow ME, Jacobson EN. J Am Chem Soc. 2002;124:1307. doi: 10.1021/ja016737l. [DOI] [PubMed] [Google Scholar]
- 7.Gaunt MJ, Hook DF, Tanner HR, Ley SV. Org Lett. 2003;5:4815. doi: 10.1021/ol035848h. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Luo Z, Chen CHT, Curran DP. J Am Chem Soc. 2002;124:10443. doi: 10.1021/ja026947d. [DOI] [PubMed] [Google Scholar]
- 9.Smith AB, III, Pitram SM, Fuertes MJ. Org Lett. 2003;5:2751. doi: 10.1021/ol034989g. [DOI] [PubMed] [Google Scholar]
- 10.Mitsunobu O. Synthesis. 1981:1. [Google Scholar]
- 11.Dess DB, Martin JC. J Org Chem. 1983;48:4155. [Google Scholar]
- 12.Blakemore PR, Browder CC, Hong J, Lincoln CM, Nagornyy PA, Robarge LA, Wardrop DJ, White JD. J Org Chem. 2005;70:5449. doi: 10.1021/jo0503862. [DOI] [PubMed] [Google Scholar]
- 13.Jung WH, Harrison C, Shin Y, Fournier JH, Balachandran R, Raccor BS, Sikorski RP, Vogt A, Curran DP, Day BW. J Med Chem. 2007;50:2951. doi: 10.1021/jm061385k. [DOI] [PubMed] [Google Scholar]
- 14.Paquette LA, Chang S. Org Lett. 2005;7:3111. doi: 10.1021/ol0511833. [DOI] [PubMed] [Google Scholar]
- 15.(a) The column is available from Fluorous Technologies, Inc. (b) D.P.C. owns an equity interest in this company.
- 16.Oishi T, Kanemoto M, Swasono R, Matsumori N, Murata M. Org Lett. 2008;10:5203. doi: 10.1021/ol802168r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.