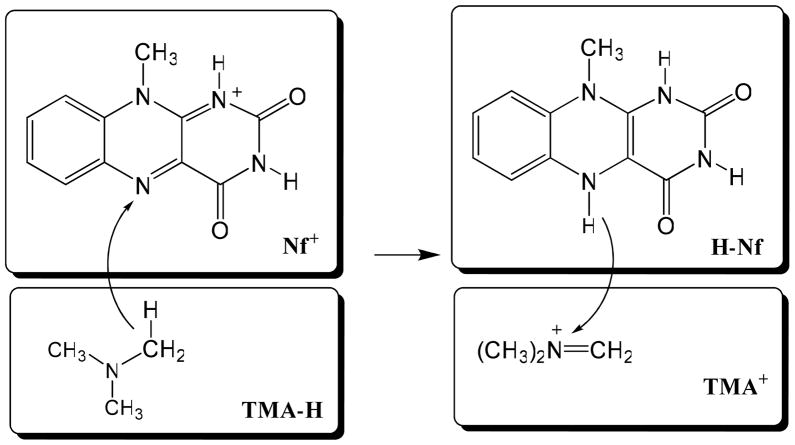
Scheme 1.
Schematic representation of the block-localization of molecular orbitals within individual molecular fragments for the reactant diabatic state (left) and the product diabatic state (right) for the hydride transfer reaction between trimethylamine (TMA-H) and a model for the flavin cofactor (Nf+). Atoms and charges in each rectangular specify the molecular block defined by the corresponding Lewis structure within which molecular molecular orbitals are localized. The antisymetric wave function constructed from the two blocks on the left-hand side of the arrow, TMA-H and Nf+, defines the reactant diabatic state, whereas that for the right-hand side blocks, TMA+ and H-Nf, define the product diabatic state.