Table 5.
Cascade AAA for the synthesis of piperazinone 19[a]
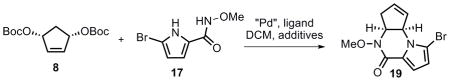 | ||||||
|---|---|---|---|---|---|---|
| Entry | Pd source (mol %) | Ligand (mol %) | Additive (mol %) | Temp. (°C) | Yield (%)[b] | Ee (%)[c] |
| 1 | [Pd(π-C3H5)Cl]2 (2) | (R,R)-LST (6) | LiHMDS (100) | rt | 0 | NA |
| 2 | [Pd(π-C3H5)Cl]2 (2) | (R,R)-LST (6) | LiHMDS (200) | rt | 0 | NA |
| 3 | [Pd(π-C3H5)Cl]2 (2) | (R,R)-LST (6) | none | rt | trace | NA |
| 4 | [Pd(π-C3H5)Cl]2 (2) | (R,R)-LST (6) | Cs2CO3 (100) | rt | 0 | NA |
| 5 | [Pd(π-C3H5)Cl]2 (5) | (R,R)-LST (15) | HOAc (10) | rt | trace [d] | NA |
| 6 | Pd2(dba)3·CHCl3 (5) | (R,R)-LST (15) | HOAc (10) | rt | 51 | NA |
| 7 | Pd2(dba)3·CHCl3 (5) | (R,R)-LST (15) | BSA (100) | rt | 50 | NA |
| 8 | Pd2(dba)3·CHCl3 (5) | (R,R)-LST (15) | HOAc (10) | 0 to rt | 65[e] | 89 |
| 9 | Pd2(dba)3·CHCl3 (5) | Rac-LST (15) | HOAc (10) | rt | 88[e] | NA |
| 10 | Pd2(dba)3·CHCl3 (5) | (R,R)-LST (15) | HOAc (10) | 0 to rt | 80[e,f] | 96 |
| 11 | Pd2(dba)3·CHCl3 (5) | (R,R)-LST (15) | HOAc (10) | 0 to rt | 82[e,g] | 97.5 |
Unless otherwise indicated, all reactions were performed with 1.0 equiv of 8 and 1.0 equiv of 17 at 0.2 M in DCM.
Isolated yield.
Enantioselectivities were determined by chiral HPLC.
Single alkylation product was the main product.
The reaction was performed with 1.5 equiv of 8 and 1.0 equiv of 17.
Another portion of Pd2(dba)3CHCl3 (5 mol %), (R,R)-LST (15 mol %) was added after 1 h.
Another portion of Pd2(dba)3CHCl3 (5 mol %), rac- LST (15 mol %) was added after 3.5 h.
