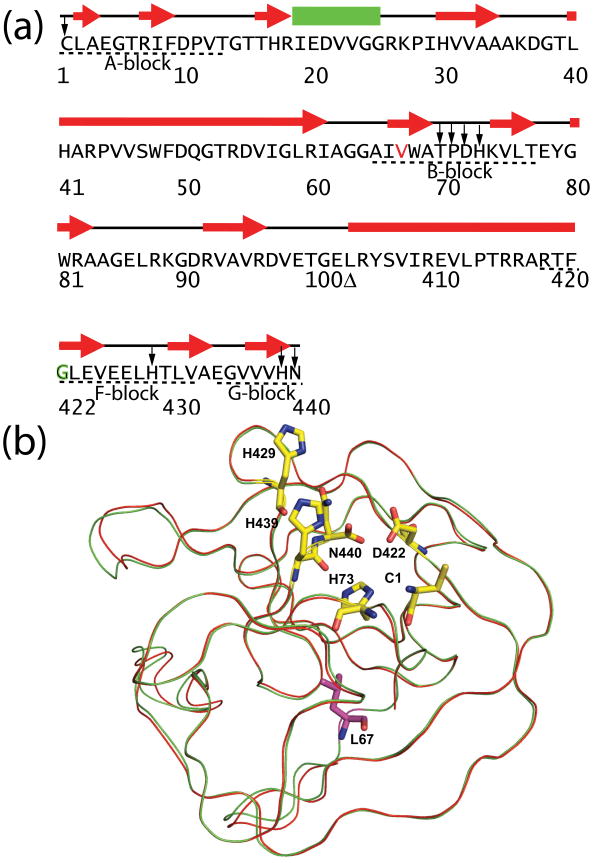
Figure 1.
Sequence and structure of minimized Mtu RecA inteins. (a) Sequences, secondary structures, and conserved blocks of engineered and minimized intein ΔΔIhh-V67CM. The residues in the minimized inteins are numbered according to the full length Mtu RecA intein, which results in a discontinuity (Δ) in residue numbers due to the deletion of the endonuclease domain and the replacement of a disordered loop. ΔΔIhh-L67CM is different from ΔΔIhh- V67CM only by the single V67L mutation (V67 colored in red). Conserved blocks are denoted by dashed lines underneath the sequence. In the CM mutant, the conserved D422 is replaced by G422 which is marked in green. Highly conserved residues are marked by arrows. (b) V67L mutation does not cause major changes in X-ray crystal structure. 3D structure of ΔΔIhh-L67SM50 is overlaid with that of ΔΔIhh-V67SM (red). Shown in stick representation (yellow) are the active site residues, C1, H73 (B-block histidine), D422 (F-block aspartate), H429 (F-block histidine), H439 (penultimate histidine) and the C-terminal residue N440. The L67 side-chain is colored purple.