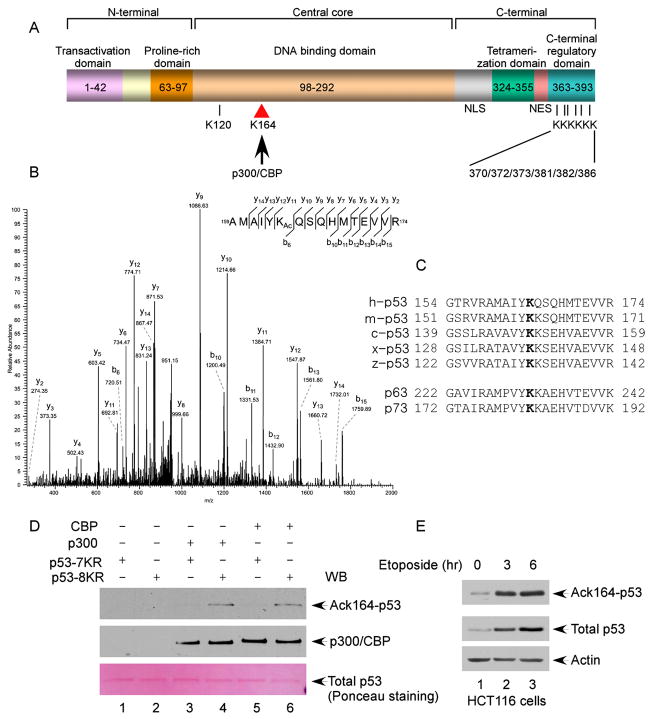
Figure 1. Identification of K164 within the Human p53 DNA-Binding Domain as a Novel Acetylation Site by p300/CBP.
(A) Schematic representation of the human p53 protein with known acetylation sites indicated, including K120, K370, K372, K373, K381, K382, and K386. In this study, K164 was identified to be acetylated by p300/CBP.
(B) Mass spectrometry analysis of the p53-derived peptides containing acetylated K164 (AcK164). The protein was prepared as described in the Experimental Procedures.
(C) Alignment of the K164 flanking region of the human p53 protein with those of p53 from other species and of human p63 and p73. The conserved lysine residue is marked in bold; h: human; m: mouse; c: chicken; x: Xenopus; and z: zebrafish.
(D) In vitro acetylation of p53 by p300/CBP. The Flag-p53-K120R/6KR (lanes 2, 4, and 6) or Flag-p53-K120R/K164/6KR (lanes 1, 3, and 5) recombinant protein was incubated alone (lanes 1 and 2), with p300 (lanes 3 and 4), or with CBP (lanes 5 and 6). The reaction products were resolved by SDS-PAGE and analyzed by western blot using the site-specific polyclonal antibody against acetylated K164 (anti-AcK164-p53) (top panel). The levels of the p53 recombinant protein substrates are shown in the bottom panel by Ponceau red staining.
(E) Acetylation of the endogenous p53 protein at K164. HCT116 cells were treated with 20 μM etoposide (0, 4, and 6 hr). The endogenous p53 proteins were immunoprecipitated by the anti-p53 (1801) antibody and assayed for acetylation by western blot using the anti-AcK164-p53 antibody (top panel). The levels of p53 in the cell extracts are shown in the middle panel.