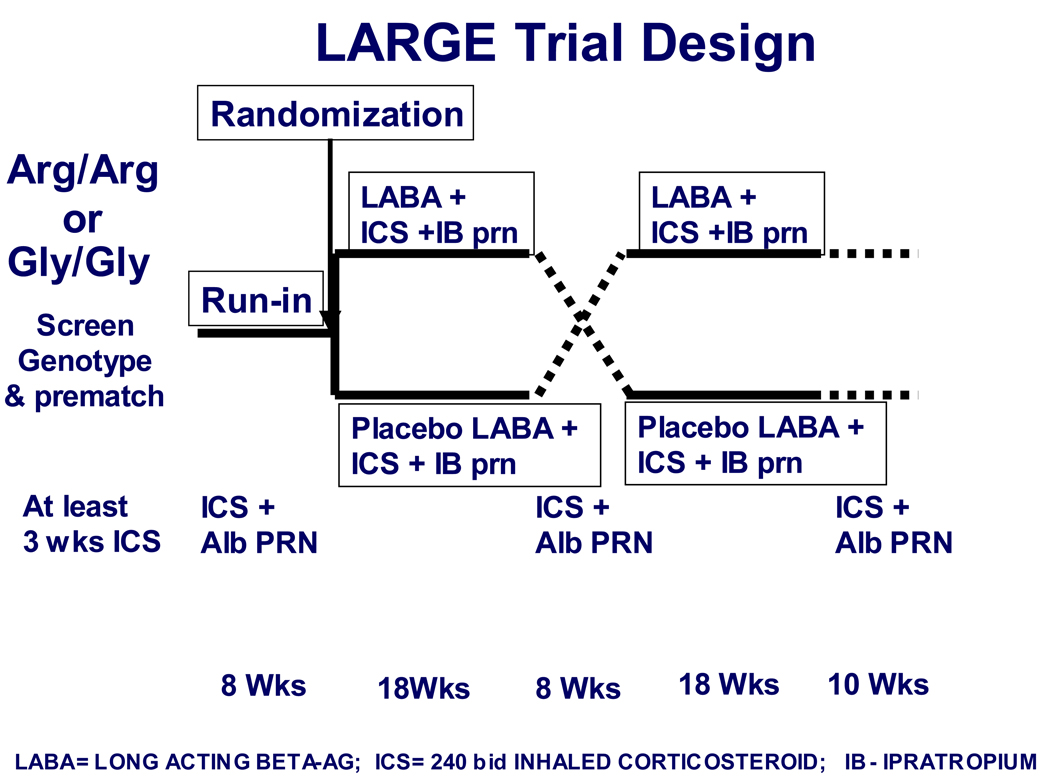
Figure 1. LARGE study design.
Following screening and genotyping, genotype-eligible and matched subjects who received 8 weeks of ICS during the run-in were randomized to continue ICS with either LABA or placebo for 18 weeks, followed by an 8 week runout period on ICS alone, followed by the alternate treatment.