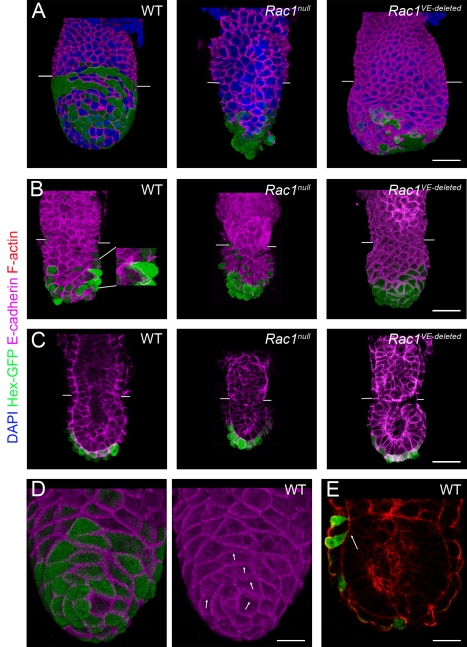
Figure 5. E-cadherin during migration.
(A, B) 3D reconstructions showing expression of Hex-GFP (green, staining with anti-GFP antibody) and E-cadherin (magenta). See Figure S2 for the separated E-cadherin channel. (A) Embryos at the time when migration should be nearly completed. Some wild-type AVE cells have reached the extra-embryonic boundary (marked by white lines in all embryos), while they remain distal in stage-matched Rac1 null and Rac1 VE-deleted embryos (dissected at e6.25). (B) e5.5–e5.75 embryos during the time of AVE migration. Wild-type and mutant Hex-GFP cells had comparable levels of E-cadherin. Migrating wild-type embryonic VE cells had diverse shapes, and some cells with long projections could be observed (inset, 2×). In Rac1 null embryos, cells were more regular and rounder. Rac1 VE-deleted embryos had a variable phenotype at e5.75. Some mutants were indistinguishable from wild-type (5/14), some had AVE cells that migrated from distal to proximal but failed to spread laterally (5/14), and some (shown) displayed a partial distal to proximal migration (4/14). (C) Individual sections from Z-stacks of e5.5–e5.75 embryos, stained for E-cadherin. Wild-type AVE cells retained adherens junctions during migration. E5.5 Rac1 null and Rac1 VE-deleted embryos displayed a single-layered epithelium with normal-appearing adherens junctions. (D) 3D reconstructions of Z-stack of a wild-type e5.5 embryo. The lateral membrane of some cells was at an oblique angle to the basement membrane, causing the E-cadherin staining to appear fuzzy (arrows). (E) Individual section from a Z-stack of a wild-type e5.75 embryo stained with phalloidin to visualize F-actin (red). AVE cells on the proximal anterior surface of the embryo are tilted in the apical/basal plane, so that their basal surfaces (arrow) are more anterior than their apical surface. Scale bars = 50 µm in A to C, and 25 µm in D and E.