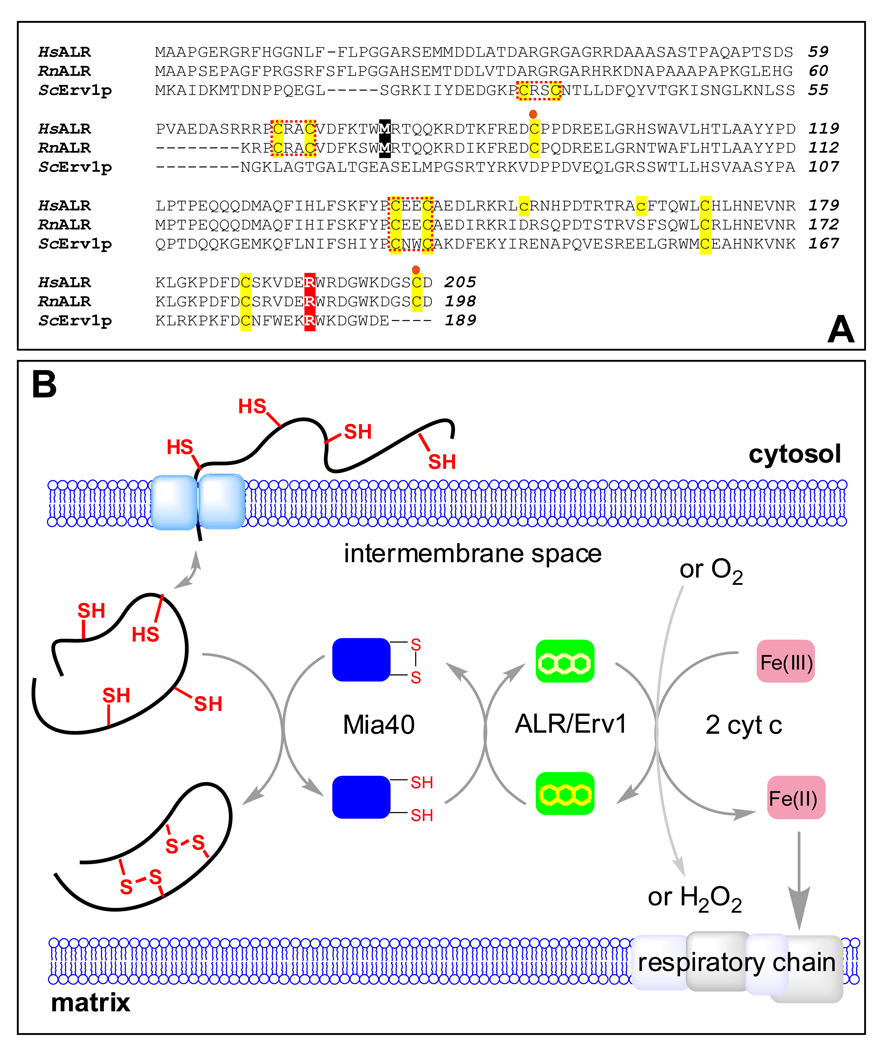
FIGURE 1.
Comparison of sequences of human and rat ALR with yeast Erv1p and a depiction of the role of ALR in oxidative folding in the mitochondrial intermembrane space. Human, rat and yeast sequences are indicated by Hs Rn and Sc prefixes respectively in the alignment shown in Panel A. All cysteines are highlighted in yellow. The N-terminal (distal) and C-terminal (proximal) redox-active CxxC motifs are enclosed within red dotted boxes. The cysteine residues forming intersubunit disulfide bonds are denoted by orange circles. The two cysteine residues in human ALR that are mutated to prevent aggregation are shown in lower case. The methionine residue highlighted in black represents the N-terminus of the short form of mammalian ALR. The position of the R194 mutant in human ALR is highlighted in red. Panel B: Proteins undergoing oxidative folding in the IMS transfer electrons to Mia40 which then transmits them to long form ALR (or Erv1p in yeast). The terminal electron acceptor is either cytochrome c or molecular oxygen.