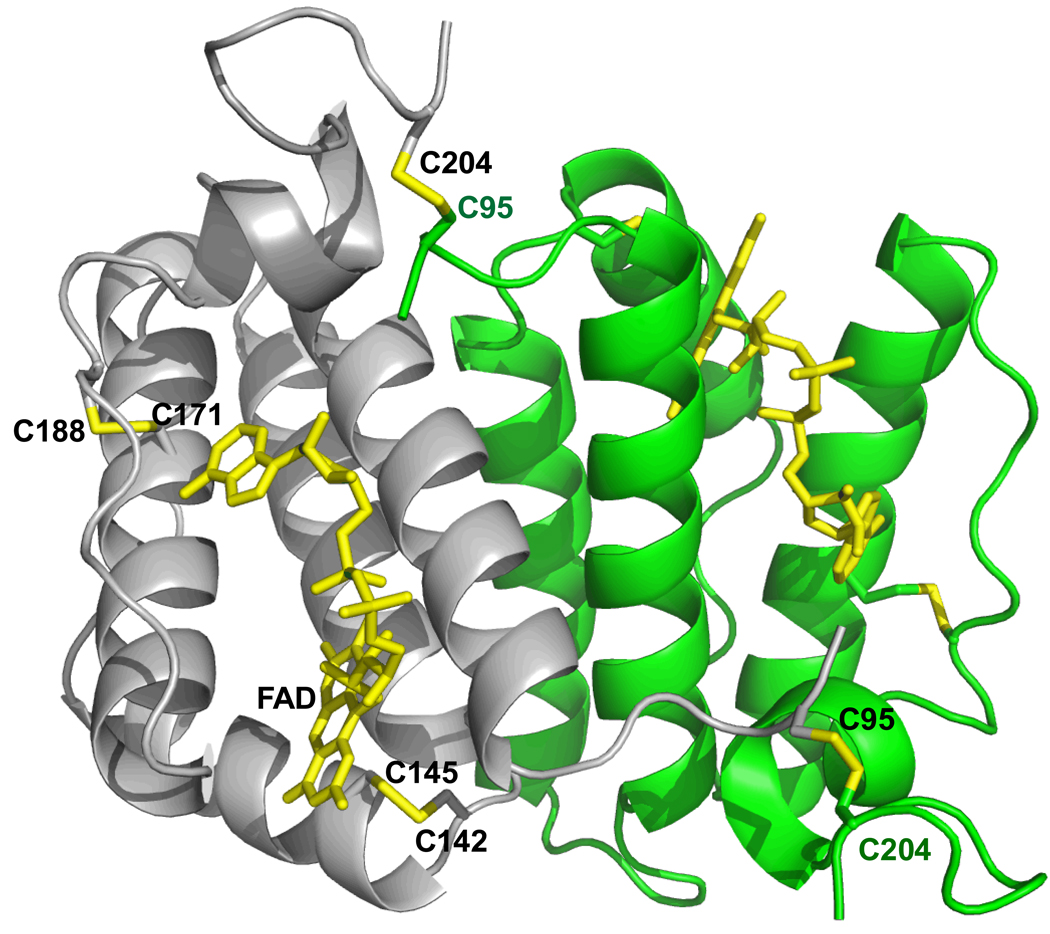
FIGURE 2.
Overall chain fold of dimeric human sfALR. The two C95-C204 disulfides that join the gray and green subunits of the sfALR homodimer are shown in yellow, together with the redox-active proximal disulfide (C142–C145) and the structural disulfide (C171–C188) in the gray subunit. The FAD is depicted in yellow.