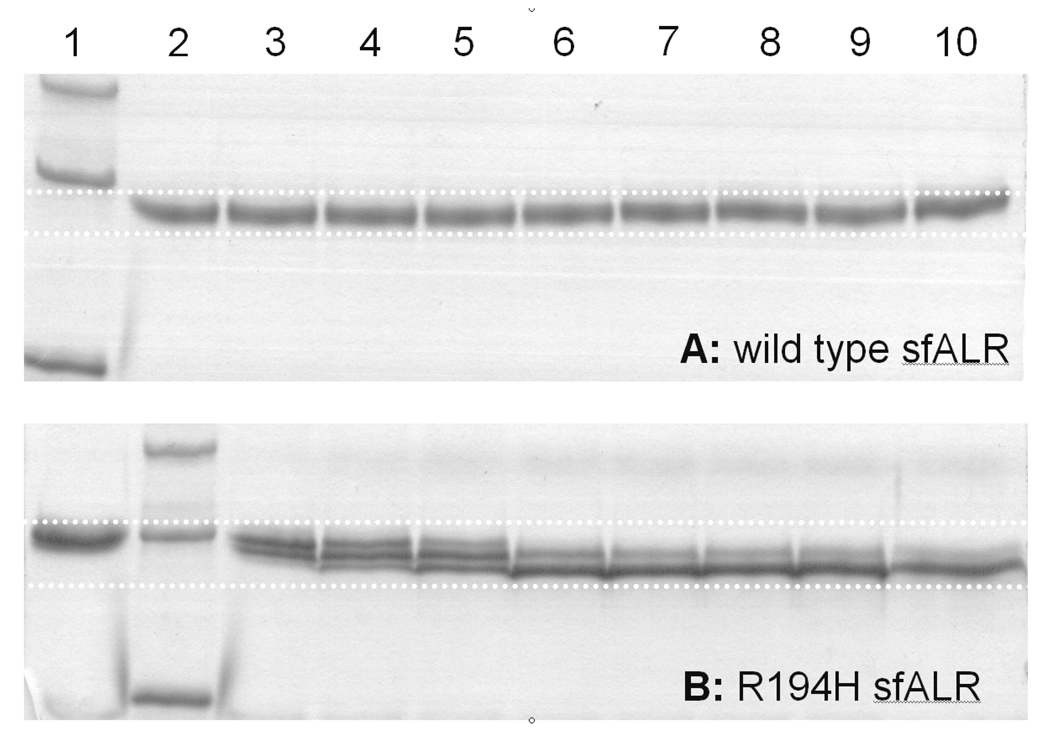
FIGURE 7.
The R194H mutant of sfALR gains sensitivity to partial proteolysis using chymotrypsin. Non-reducing SDS-PAGE gels were run with protein standards (35, 25 and 15 kDa from the top of panel A, lane 1; and panel B lane 2). R194H (lane 1 panel B) reproducibly runs slightly slower than wild type protein (lane 2, panel A) on SDS-PAGE. Proteins were treated with 1% w/w chymotrypsin in 50 mM phosphate buffer, pH 7.5, 25 °C, containing 0.3 mM EDTA, and samples were quenched in non-reducing Laemmli buffer (for panel A, lanes 2–10 were quenched 0, 1, 3, 5, 10, 15, 30, 60 and 120 min after protease treatment of sfALR). For the mutant in panel B, lane 1 is at time zero, lane 2 is the protein ladder and lanes 3–10 reflect the same time intervals as in panel A. For clarity, the dotted lines on the gels define the limits of band migration.